Phase III data in metastatic NSCLC support an alternative route of administering immunotherapy, which may have an impact on patients’ preferences and cancer care costs
Subcutaneous (SC) pembrolizumab – consisting of pembrolizumab co-formulated with berahyaluronidase alfa – demonstrated noninferiority in first cycle area-under-the-curve (AUC) exposure and steady-state trough concentrations compared with intravenous (IV) pembrolizumab in the phase III MK-3475A-D77 trial presented at the European Lung Cancer Congress 2025 (Paris, 26–29 March) (Abstract 8MO).
As reported in the study, geometric mean ratios were 1.14 (96% confidence interval [CI] 1.06–1.22) for AUC0-6wk for cycle 1 and 1.67 for steady-state Ctrough (94% CI 1.52–1.84), which were above the noninferiority margin of 0.8 (both p<0.0001). The trial involved 377 patients with newly diagnosed metastatic non-small cell lung cancer (NSCLC) without sensitising mutations who were randomised 2:1 to pembrolizumab SC 790 mg or pembrolizumab IV 400 mg every 6 weeks in addition to platinum doublet chemotherapy.
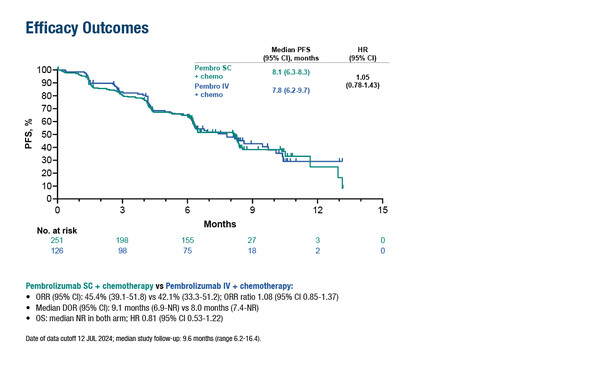
Over a median follow-up of 9.6 months, objective response rates were 45.4% with SC and 42.1% with IV, while median progression-free survival was 8.1 months and 7.8 months (hazard ratio 1.05; 95% CI 0.78–1.43), respectively, and median overall survival was not reached in either arm. Grade ≥3 drug-related adverse events (AEs) were reported in 47.0% of patients in the SC group and 47.6% in the IV group, with similar rates of discontinuations due to drug-related AEs (8.4% versus 8.7%, respectively). In total, 2.4% of patients had injection-site AEs with pembrolizumab SC.
Commenting on the findings, Prof. Rosalyn Juergens from McMaster University, Hamilton, ON, Canada, says: “There have been positive results and approvals with other SC immunotherapy agents, such as atezolizumab (Ann Oncol. 2023;34:693–702) and nivolumab (Ann Oncol. 2024;35(Suppl 2):S1013–S1014); however, data from the MK-3475A-D77 trial are particularly important given how frequently pembrolizumab is used to treat NSCLC and other tumour types.”
Not only could the SC administration of immunotherapy potentially reduce the number and duration of outpatient visits, thus representing a preferred option for patients over IV therapy (ESMO Open. 2024;9(Suppl 3):102706), but it may also come with considerable financial advantages in resource-constrained healthcare settings. “As patients with lung cancer are living longer and receiving treatment for many years, efficiencies must be found,” comments Juergens, who points out that SC administration is linked to reductions in pharmacy preparation, nursing administration time, infusion-chair occupancy and fewer infusion-related AEs than with IV administration (Expert Rev Pharmacoecon Outcomes Res. 2023;23:1017–1026). “However, indirect cost savings may need to be assessed and balanced with potentially higher costs related to the higher dose of pembrolizumab within the SC formulation.”
Programme details
Felip E, et al. Subcutaneous (SC) versus intravenous (IV) pembrolizumab (pembro) plus chemotherapy (CT) in metastatic non-small cell lung cancer (mNSCLC): Phase III MK-3475A-D77 trial. European Lung Cancer Congress 2025, Abstract 8MO
Mini Oral Session 1, 27.03.2025, h. 16:00 – 17:05, Room South Paris