Studies evaluate the efficacy and safety of promising agents including adagrasib and fulzerasib
Presentations at the European Lung Cancer Congress (ELCC) 2025 (Paris, 26–29 March) provide novel insights into the treatment of patients with non-small cell lung cancer (NSCLC) harbouring the KRASG12C mutation, which occurs in approximately 10–13% of advanced non-squamous NSCLC cases (Lung Cancer. 2023;184:107293).
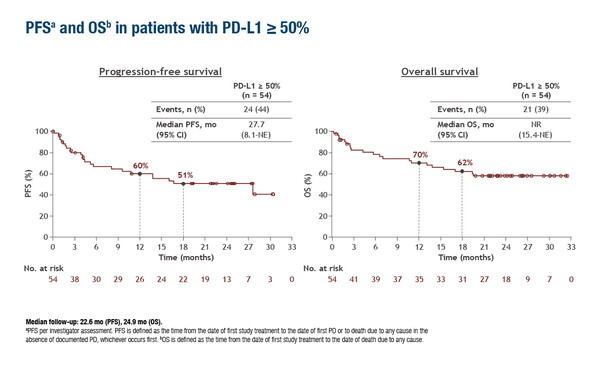
According to updated data from the phase II KRYSTAL-7 trial, the combination of adagrasib plus pembrolizumab continued to have encouraging efficacy in the first-line treatment of 54 patients with advanced/metastatic KRASG12C-mutated NSCLC and PD-L1 ≥50% (Abstract 5MO). The objective response rate (ORR) was 59% and median duration of response (DOR) was 26.3 months. With median follow-up of more than 22 months, median progression-free survival (PFS) was 27.7 months and the 18-month overall survival (OS) rate was 62%.
“The addition of adagrasib to pembrolizumab showed an impressive prolongation of PFS when compared with the standard of care, pembrolizumab monotherapy, which, based on the KEYNOTE-024 trial is associated with median PFS of around 10 months (N Engl J Med. 2016;375:1823–1833),” says Dr Adrianus Johannes De Langen from the Netherlands Cancer Institute, Amsterdam, Netherlands. However, he notes the high levels of toxicity seen with the combination. Across all 149 patients in the KRYSTAL-7 trial, regardless of PD-L1 score, 68% reported grade ≥3 treatment-related adverse events (TRAEs). Three patients experienced grade 5 TRAEs: pneumonia (n=2) and pneumonitis (n=1). TRAEs led to discontinuation of adagrasib only in 7% of patients, pembrolizumab only in 17% of patients and both adagrasib and pembrolizumab in 7% of patients.
“The rate of grade ≥3 TRAEs with adagrasib plus pembrolizumab was considerably higher than the rate of 27% seen with pembrolizumab monotherapy in the KEYNOTE-024 trial,” comments De Langen. “The high toxicity levels may mean that this combination will only be tolerated by the fittest patients.” This is now being tested as the KRYSTAL-7 trial moves into phase III where adagrasib plus pembrolizumab is being compared against pembrolizumab monotherapy in patients with KRASG12C-mutated NSCLC and PD-L1 ≥50% (NCT04613596).
Also reported in Paris were updated results from the phase II KROCUS trial investigating fulzerasib in combination with cetuximab in 47 patients with previously untreated advanced KRASG12C-mutated NSCLC (LBA1). The ORR was 80%, with 58% of patients exhibiting ≥50% tumour shrinkage. Median PFS was 12.5 months, with median DOR not reached after median follow-up of 12.8 months. Responses were consistent across levels of PD-L1 expression. Regarding tolerability, grade 3 TRAEs were reported in 14.9% of patients, most commonly rash (2%) and asthenia (2%), with no grade 4 or 5 TRAEs. In total, 6.4% of patients discontinued treatment due to TRAEs. The researchers noted that a phase III trial with fulzerasib plus cetuximab versus pembrolizumab plus chemotherapy is being planned in untreated patients with KRASG12C-mutated NSCLC and PD-L1 <50%.
“Short-term efficacy in terms of ORR across different PD-L1 levels and the safety profile appear very good, but this is the first time PFS data have been presented for fulzerasib plus cetuximab. The duration seems to be limited as compared to adagrasib and pembrolizumab combination treatment,” remarks De Langen, who thinks that adding immunotherapy to the dual combination may help to improve long-term response.
De Langen concludes by highlighting that this is an “extremely crowded area of research, so the treatment scenario for patients with KRASG12C-mutated NSCLC may be expected to evolve in the near future.” Many first-line phase III combination trials are ongoing, including some with novel KRASG12C inhibitors such as olomorasib (NCT06119581), divarasib (NCT06793215) and MK-1084 (NCT06345729).
At ELCC 2025, encouraging and durable clinical activity was also reported for daraxonrasib, an oral, RAS(ON), multi-selective, tri-complex inhibitor of GTP-bound mutant and wild-type RAS, in patients with previously treated, locally advanced or metastatic RAS-mutant NSCLC (Abstract 6MO). In the phase I/Ib, multicentre open-label study, ORR was 38%, median DOR was 15.5 months, median PFS was 9.8 months and median OS was 17.7 months. TRAEs led to dose modifications in 41% of patients and to dose discontinuations in 4% of patients, with rash (7%) being the only grade 3 TRAE in ≥5% patients and no grade 4 or 5 TRAEs observed.
Programme details
Garassino MC, et al. First-line adagrasib (ADA) with pembrolizumab (PEMBRO) in patients (pts) with advanced/metastatic KRASG12C-mutated non-small cell lung cancer (NSCLC) and PD-L1 ≥50% from the phase II portion of KRYSTAL-7. European Lung Cancer Congress 2025, Abstract 5MO
Mini Oral Session 1, 27.03.2025, h. 16:00 – 17:05, Room South Paris
Majem Tarruella M, et al. First-line (1L) fulzerasib + cetuximab in KRAS G12Cm advanced NSCLC: updated efficacy and safety from KROCUS study. European Lung Cancer Congress 2025, Abstract LBA1
Mini Oral Session 1, 27.03.2025, h. 16:00 – 17:05, Room South Paris
Punekar S, et al. Safety and clinical activity of daraxonrasib (RMC-6236) in RAS mutant non-small cell lung cancer (NSCLC). European Lung Cancer Congress 2025, Abstract 6MO
Mini Oral Session 1, 27.03.2025, h. 16:00 – 17:05, Room South Paris