Immunotherapy typically requires intravenous administration, but this may not be the optimal or preferred route for all patients
Immunotherapy with nivolumab, a PD-1 inhibitor, is approved for intravenous (IV) administration; however, other routes of administration might have advantages and could meet different patient preferences or needs and are important to explore.
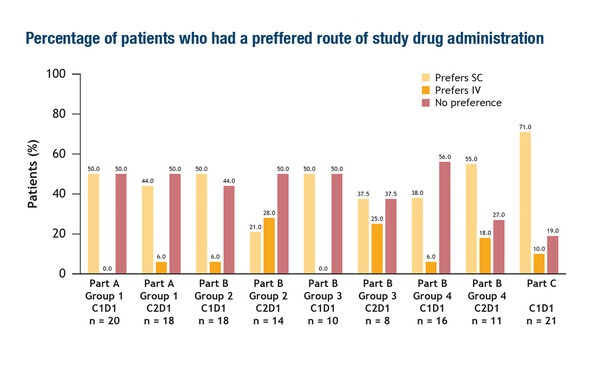
Two presentations at ESMO Congress 2022 focussed on this issue. Firstly, an exploratory analysis of the phase I/II CheckMate 8KX study of subcutaneous (SC) nivolumab with or without recombinant human hyaluronidase PH20 enzyme (rHuPH20) in patients with advanced or metastatic tumours surveyed patient preferences for SC or IV nivolumab (Abstract 739P). Over the course of different treatment cycles, more patients declared a preference for SC over IV nivolumab (21–71% versus 0–28%, respectively) and reported minimal pain/discomfort after SC injection.
Added to this, from the first reported trial data, we can see that nivolumab exposure increased with SC dose, with lower maximum serum concentration (Cmax) but higher serum concentration at the end of the dosing interval (Ctau) than historical values with IV nivolumab. In addition, the safety profile of SC nivolumab was consistent with IV nivolumab (J Clin Oncol. 2021;39[Suppl 15]:2575). These early data are encouraging but not unexpected, given that conventional antibodies have been safely administered via the SC route. However, regardless of patient preferences, we need more robust data to show equivalence in terms of efficacy and safety, particularly with regards to the different pharmacokinetics, as these aspects will always be our foremost concerns.
Utilising an SC route of administration could potentially reduce the number and duration of outpatient visits, but on the flip side, can also come with potential issues. In some countries for example, reimbursement of procedures by the healthcare system is linked to the route of administration, and is lower with SC than IV treatments. Therefore, there may be limited incentive for physicians to implement SC regimens in the absence of a clear clinical rationale. Additionally, patient-related factors such as body weight and extent of SC tissue may impact the tolerability of such treatment.
A second abstract presented at the Congress reported the safety and preliminary efficacy findings from a phase I study of three doses of intraperitoneal (IP) nivolumab after cytoreductive surgery (CRS) and hyperthermic IP chemotherapy (HIPEC) in 10 patients with advanced ovarian cancer (Abstract 614P). No dose-limiting toxicity – the primary objective – was demonstrated at any dose. Nine patients had severe adverse events, but these were generally of the type that occur with CRS or HIPEC, and none were considered to be related to IP nivolumab or were immune-related. At a median follow-up of 10.1 months, median progression-free survival was 7.4 months (95% confidence interval 6.0–not yet reached). Despite this being an early study in a small number of patients, evidence suggesting that IP nivolumab does not add to the burden of treatment is a promising result and adds to potential application of checkpoint inhibitors from a pharmacological perspective. However, my belief is that there are too many outstanding questions for these results to be game changing. Conceptually and from a pharmacokinetic (PK) perspective, several caveats apply to these data. In many in vivo models, the PK of IP treatment with antibody-based therapy is similar, just delayed, compared with IV treatment, therefore, benefits over systemic treatment are unclear. Moreover, IP treatment might not be the best route for all patients but could rather be an interesting option for some that require IP intervention to reduce the amount of procedures to be applied in a palliative setting. Larger studies and those in patients earlier in their disease course need to go some way to address these concerns.
Abstracts discussed:
Lonardi S, et al. Patient preference for subcutaneous nivolumab (NIVO) with/without recombinant human hyaluronidase PH20 (rHuPH20) vs intravenous NIVO: an exploratory analysis of a phase 1/2 pharmacokinetic multi-tumour study. ESMO Congress 2022, Abstract 739P
Poster display, Hall 4. An e-Poster is also available on the Congress virtual platform
Corbaux P, et al. Tolerance and preliminary efficacy of intraperitoneal (ip) nivolumab after cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients (pts) with advanced ovarian carcinoma: a phase I study with expansion cohort (ICONIC). ESMO Congress 2022, Abstract 614P
Poster display, Hall 4. An e-Poster is also available on the Congress virtual platform