Results from the ETOP AMAZE-lung and COCOON trials show some promise to improve safety and reduce skin adverse events
As the standard of care for patients with EGFR-mutant advanced non-small cell lung cancer (NSCLC) evolves, international research efforts continue to try to balance efficacy and toxicity, as illustrated by two studies presented at the European Lung Cancer Congress 2025 (Paris, 26–29 March).
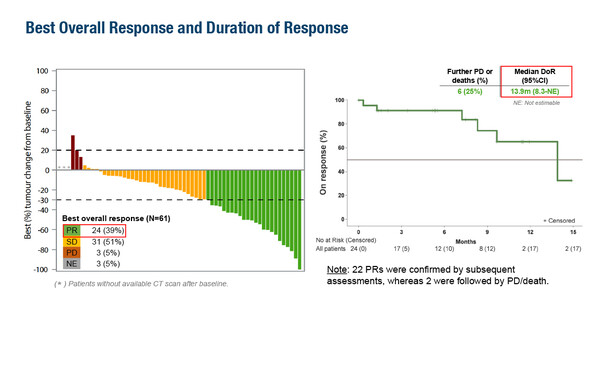
The single-arm phase II ETOP AMAZE-lung trial sought to provide an alternative to amivantamab plus chemotherapy for patients after progression on osimertinib and demonstrated modest yet durable efficacy with the combination of amivantamab, lazertinib and bevacizumab (Abstract 9MO). The primary endpoint of confirmed objective response rate (ORR) was 33% at 12 weeks among 60 patients in the primary efficacy cohort, which met with the study’s pre-defined success criterion of ORR >20%. Among 61 patients evaluated, the best overall response was stable disease in 51%, partial response in 39%, disease progression in 5% and not evaluable in 5%, with median duration of response of 13.9 months (95% confidence interval [CI] 8.3–not estimable). Median progression-free survival was 10.9 months (95% CI 5.3–not estimable) and median overall survival (OS) was 15.5 months (95% CI 12.3–not estimable).
In total, 43% of patients experienced grade 3–4 treatment-related adverse events (TRAEs) and one-fifth of patients discontinued any agent due to TRAEs. The most common grade 3–4 TRAEs were acneiform and maculo-papular rash.
“Overall, the efficacy results were not remarkable but they were long lasting,” says Prof. Julien Mazières from Toulouse University Hospital, Toulouse, France. “It is reassuring that bevacizumab did not appear to add significant toxicity to that of amivantamab plus lazertinib. The triple combination seems to represent a reasonable chemotherapy-free alternative, but we need more mature data with larger patient numbers and ideally, a head-to-head randomised trial versus amivantamab plus chemotherapy,” he adds.
Results were also presented from the COCOON trial, which found a significantly reduced incidence and severity of skin AEs when using a prophylactic enhanced dermatologic regimen compared with standard dermatologic management following the initiation of amivantamab plus lazertinib for the first-line treatment of EGFR-mutated advanced NSCLC (Abstract 10MO). The COCOON regimen comprised oral doxycycline or minocycline, topical clindamycin lotion on the scalp, chlorhexidine on the nails, and ceramide-based moisturiser on the body and face. At the first pre-planned interim analysis (median follow-up of 4.2 months), the primary endpoint of incidence of grade ≥2 dermatologic AEs in the first 12 weeks was met. There was a significant halving of grade ≥2 dermatologic AEs from 76.5% with standard dermatologic management to 38.6% with the COCOON regimen (p<0.0001), the greatest (~70%) reduction being reported for scalp AEs (29% versus 9%). There was also a reduction in grade 3 dermatologic AEs, from 8.8% with standard management to 4.3% with the COCOON regimen. Importantly, rates of AE-related amivantamab and lazertinib discontinuations were lower with the COCOON regimen than with standard management, both for dermatologic AEs (1% versus 4%) and any AEs (11% versus 19%).
“Traditionally, skin reactions have been managed reactively, once they emerge, but having an effective regimen that can be used prophylactically may allow patients to stay on treatment longer. This is particularly important given that use of amivantamab plus lazertinib is expected to increase with the release of final OS data from the MARIPOSA trial at the Congress ,” observes Mazières. However, he remains cautious about the COCOON regimen owing to the relatively high dose of doxycycline/minocycline (100 mg twice daily) compared with the standard dose in many countries, and the prolonged duration of the regimen (12 weeks).
“There are many combination regimens currently undergoing clinical trials for the treatment of EGFR-mutant advanced NSCLC, including the combination of tyrosine kinase inhibitor (TKI) plus anti-angiogenic agents, TKI plus chemotherapy, or bispecific antibody, such as amivantamab, plus chemotherapy, but with each combination we must deal with different toxicities. The key is to be aware of how to prevent, detect and treat these toxicities as early as possible,” concludes Mazières.
Programme details
Soo RA, et al. Amivantamab, lazertinib and bevacizumab in EGFR-mutant advanced NSCLC with acquired resistance to 3rd generation EGFR-TKI - Final results from the phase II ETOP AMAZE-lung trial. European Lung Cancer Congress 2025, Abstract 9MO
Mini Oral Session 1, 27.03.2025, h. 16:00 – 17:05, Room South Paris
Girard N, et al. Preventing moderate to severe dermatologic adverse events in first-line EGFR-mutant advanced NSCLC treated with amivantamab plus lazertinib: Early success of the COCOON trial. European Lung Cancer Congress 2025, Abstract 10MO
Mini Oral Session 1, 27.03.2025, h. 16:00 – 17:05, Room South Paris