Two studies add further evidence of the advantages of this route for delivering immune checkpoint inhibitors for patients and healthcare sustainability
With reductions in infusion-related reactions, clinic visits and time, together with patient preference, subcutaneous (SC) administration may be the future of immunotherapy, as suggested by two studies presented at the European Lung Cancer Congress 2024 (Prague, Czech Republic, 20–23 March).
The phase Ib PALOMA study previously reported that SC administration of amivantamab once every two weeks (Q2W) or three weeks (Q3W) to patients with various solid tumours was better tolerated than intravenous (IV) administration, with a lower incidence of infusion-related reactions (IRRs) (16% versus 67%, by indirect comparison) (J Clin Oncol. 2023;41(16_Suppl);9126). A further analysis from this study now shows that the immunotherapy can be delivered SC every four weeks (Q4W) without compromising efficacy (Abstract 6MO). In the 19 patients treated, the IRR incidence of 16% was identical to that observed previously; all reactions were grade 1–2 and occurred after the first dose only. A Q4W dose of 3,520 mg (≥80 kg, 4,640 mg) had similar exposure to the approved IV dose, with simulated geometric mean ratios compared to the reference IV dose at steady state of 0.92 (90% confidence interval [CI] 0.76–1.11) for trough concentration and 1.27 (90% CI 1.18–1.36) for area under the concentration–time curve from 0 to 672 hours.
“The fact that treatment can be given successfully for 7 minutes every 4 weeks instead of 2 to 4 hours every 2 or 3 weeks – with similar exposure to avoid compromising efficacy – is a major benefit,” explains Prof. Benjamin Besse from Institut Gustave Roussy, Villejuif, France. “Helping patients to have a life as normal as possible is one of our aims and yet frequent infusions are a huge burden on patients and caregivers, particularly as many patients now receive treatment for years and some patients travel miles for each hospital visit. One could speculate that reduced frequency SC administration may even increase treatment uptake.” The reduction in the incidence of IRRs was substantial with SC administration and, as these unpleasant reactions are also often seen at rechallenge, Besse notes that the benefits of reducing IRRs may not just apply to the first administration.
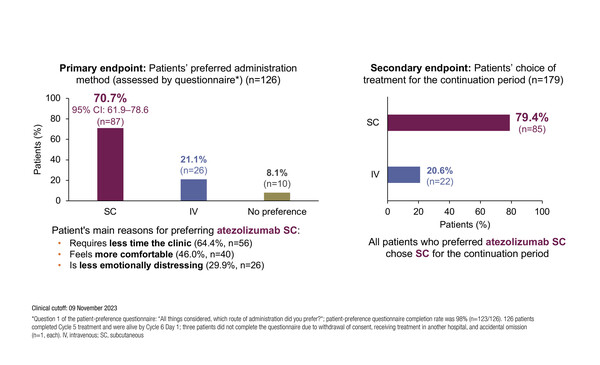
In addition to its potential benefits, previous studies reported that SC administration is preferred by patients, and confirmation is also given by the results of the IMscin002 cross-over study presented at ELCC 2024 (Abstract 244MO). The primary endpoint was met, such that 70.7% of patients reported a preference for atezolizumab SC compared with 21.1% for atezolizumab IV after three cycles of each. The main reasons for the preference were the shorter time needed in the clinic (64.4%), SC administration felt more comfortable (46.0%) and less emotionally distressing (29.9%). After three cycles of each, patients selected their preferred route for the continuation period and more than three-quarters (79.4%) chose SC administration. In total, 85.8% of patients reported being very satisfied or satisfied with atezolizumab SC compared with 75.2% with atezolizumab IV, with no new safety concerns identified.
“The robust methodology of this study, whereby all patients experienced three cycles each of atezolizumab SC and IV Q3W before being allowed to select the preferred route for the remainder of their treatment, adds weight to the findings,” says Besse. One of the biggest challenges facing SC delivery may be pain caused by the volume of agent being administered. “However,” says Besse, “de-escalation of immunotherapy dose is constantly being investigated in the IV setting and this may lead in the future to lower doses, and reduced volumes, to facilitate SC delivery.”
Last year, results from the IMscin001 study showed that SC and IV atezolizumab had comparable exposure, efficacy and safety in patients with non-small cell lung cancer (Ann Oncol. 2023;34:693–702). This led to NHS England’s world-first rollout of an SC anti-PD-(L)1 agent, heralded as cutting treatment time by up to 75% (NHS England). Besse highlights that positive findings from studies may further encourage the investigation of alternative routes of administration of other immune checkpoint inhibitors, with a potential impact on the sustainability of healthcare services. The shorter treatment time afforded by SC administration has implications for healthcare utilisation, including reduced patient bed/chair time and shorter active time by healthcare professionals (HCPs) (Future Oncol. 2019;15:3267–3281). “We have a shortage of nurses and increasing numbers of patients needing treatment,” observes Besse. “Shorter preparation time, administration time and time for monitoring IRRs could improve sustainability, allowing HCPs to treat more patients in any given day.”
Abstracts discussed:
Leighl N, et al. Subcutaneous amivantamab administered every 4 weeks (Q4W) in patients with advanced solid malignancies: The phase 1b PALOMA study. European Lung Cancer Congress 2024, Abstract 6MO
Mini Oral Session 2, 21.03.2024, h. 17:00 – 17:50, Forum Hall
Cappuzzo F, et al. Primary results from IMscin002: A study to evaluate patient (pt)- and healthcare professional (HCP)-reported preferences for atezolizumab (atezo) subcutaneous (SC) vs intravenous (IV) for the treatment of NSCLC. European Lung Cancer Congress 2024, Abstract 244MO
Mini Oral Session 2, 21.03.2024, h. 17:00 – 17:50, Forum Hall