However, secondary endpoints of the TROPION-Breast01 continue to support a potential benefit of the antibody-drug conjugate in previously treated patients
Final analysis data from the TROPION-Breast01 trial provides novel evidence of the potential clinical value of datopotamab deruxtecan (Dato-DXd), a TROP2-directed antibody-drug conjugate (ADC), for the treatment of women with hormone receptor–positive/human epidermal growth factor receptor 2–negative (HR+/HER2–) breast cancer (VP1-2025). As reported at today’s ESMO Virtual Plenary, despite overall survival (OS) results did not reach statistical significance, the manageable safety profile and secondary efficacy endpoints continued to favour Dato-DXd versus investigator's choice of chemotherapy (ICC) in the study.
The final analysis follows the positive progression-free survival (PFS) results from the randomised phase III trial that were firstly presented at the ESMO Congress 2023 demonstrating a statistically significant and clinically meaningful improvement in PFS by blinded independent central review (BICR) with Dato DXd (6 mg/kg once every three weeks) compared with ICC (eribulin/vinorelbine/capecitabine/gemcitabine) in patients with previously treated, inoperable or metastatic HR+/HER2– breast cancer (JCO 43, 285-296(2025)). In the study, a total of 732 patients were randomly assigned to either the ADC arm (n=365) or the ICC arm (n=367), the dual primary endpoints were PFS by BICR and OS, while secondary endpoints included PFS by investigator and progression-free survival 2 (PFS2).
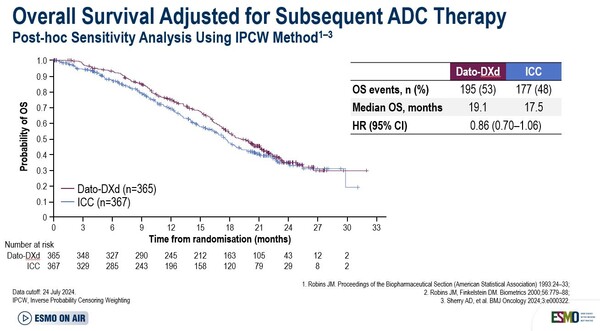
As presented at the ESMO Virtual Plenary, at a median follow-up of 22.8 months OS for the Dato-DXd versus the ICC arm did not reach statistical significance (HR 1.01 [95% CI 0.83–1.22]; p=0.94). “A potential explanation for the lack of impact in OS may be that the trial was unpowered to detect OS differences. Also, findings may have been influenced by the subsequent therapy and crossover”, says Dr Véronique Diéras from the Centre Eugène Marquis, Rennes, France, commenting on the results. OS sensitivity analysis for subsequent ADC use showed an adjusted HR of 0.86 (95% CI 0.70‒1.06) indicating that subsequent treatment following patients’ disease progression or treatment discontinuation is likely to have affected survival results (Figure).
“During the course of the TROPION-Breast01 study, two ADCs were approved based on the results from the DESTINI-Breast04 study (N Engl J Med. 2022 Jul 7;387(1):9-20) and the TROPICS-02 (Lancet. 2023 Oct 21;402(10411):1423-1433): trastuzumab deruxtecan (T-DXd) for HR+/HER2 low metastatic breast cancer after one or two lines of chemotherapy and sacituzumab govitecan (SG) for HR+/HER2 metastatic breast cancer after two lines of chemotherapy, respectively. In TROPION-Breast01, the use of other ADCs as subsequent therapy after discontinuation of the study treatment was imbalanced: twice in the control arm,” she highlights.
Experts agree that the study secondary endpoints are important to evaluate the potential benefits of Dato-DXd in this setting. “PFS2 and time to first and second subsequent treatments (TFST; TSST) were all delayed in the Dato-DXd arm indicating that the benefits of this ADC compared to ICC extended beyond the first progression”, continues Diéras. “Also, the patient-reported outcomes reported delayed time to deterioration (TTD) in Global Health Status/Quality of Life (GHS/QOL) in favour of Dato-DXd with 9.0 versus 4.8 months in the ADC versus ICC arm, respectively (HR 0.76; 95% CI 0.58-0.98).”
The overall safety summary including additional ~12 month of follow-up was consistent with additional safety findings of Dato-DXd compared with ICC that were presented at the ESMO Breast Cancer last year, showing that adverse events of special interest were generally low grade, easily managed and did not compromise scheduled treatment. In the final analysis, the rate of grade ≥3 treatment-related adverse events (TRAEs) in the ADC group was less than half that what observed with chemotherapy, leading to fewer dose reductions or interruptions. Rates of TRAEs leading to discontinuation were similar between arms.
“Dato-DXd may be a new treatment option for patients with advanced HR+/HER2-. However, considering the major improvements in terms of PFS and OS observed in the DESTINY04 trial, T-DXd should be the first treatment option for patients with a HER2 low status and no other contraindications (e.g. pneumonitis)”, concludes Diéras. “No data support a comparison between the use of Dato-DXd and SG as in TROPICS-02 patients were heavily pretreated. The differences in ADCs in terms of antibody target, payload, linker and drug-antibody ratio may lead to variations in efficacy and/or safety profile. This study highlights the need for biomarkers to select the best treatment sequencing for patients.”