Early-phase clinical trial results show promise to optimise the current standard of care by using dual-targeting agents and investigating the most effective chemotherapy backbone for PD-L1 blockade
Research in triple-negative breast cancer (TNBC) continues to identify the next leap forward for first-line treatment after the approval of pembrolizumab in combination with chemotherapy for high-expressing PD-L1 disease (US FDA approval, 2021), and studies presented at the ESMO Congress 2024 (Barcelona, 13–17 September) provide new insights.
Results of two novel targeted agents in combination with chemotherapy were presented for the treatment of women with advanced TNBC.
Researchers from China reported results from a single-arm, open-label, multicentre, phase II study of first-line PD-1- and VEGF-targeting ivonescimab plus paclitaxel or nab-paclitaxel in 30 patients with locally advanced unresectable or metastatic TNBC (Abstract 347MO). At a median follow-up of 10.1 months, the objective response rate (ORR) was 72.4% and the disease control rate (DCR) was 100%. Investigating the impact of PD-L1 combined positive score (CPS) status, patients with CPS <10 – who would currently be treated solely with chemotherapy – had an ORR of 69.6%, while pembrolizumab-eligible patients (with CPS ≥10) had an ORR of 83.3%. The 6-month progression-free survival (PFS) rate was 73.3%, median PFS was 9.3 months and overall survival is not yet mature. Adverse events (AEs) appeared to be similar to those expected with chemotherapy. Commenting on these results, Dr Sherene Loi from the Peter MacCallum Cancer Centre, Melbourne, Australia, says, “Although these initial results are encouraging, the mature data will be critical to aid interpretation and are awaited with interest. Further randomised studies that compare against the current standard of care – as well as studies in broader patient ethnicities – will help us ultimately determine the value of ivonescimab in the treatment of TNBC.”
A second Chinese research group reported the results of a phase Ib/II, single-arm study of first-line PM8002/BNT327 – another bispecific antibody targeting PD-L1 and VEGF – plus nab-paclitaxel in patients with locally advanced or metastatic TNBC (Abstract 348MO). At data cut-off after a median follow-up of 16.3 months, median PFS was 13.5 months, the DCR was 95.2% and the confirmed ORR was 73.8% in the 42 enrolled patients. All patients reported treatment-related AEs, with 57.1% having grade ≥3 events and 35.7% having immune-related AEs. Loi notes, “The very impressive ORR appeared to be independent of CPS score; however, patient numbers in the CPS <10 versus ≥10 groups were small, but as with the study of ivonescimab plus chemotherapy, these findings need to be confirmed in a larger preferably randomised study.”
Reflecting on the first-line treatment of advanced TNBC, Loi thinks that the findings of these and other combination studies will be highly valuable in understanding how we can improve on the current standard of care. “Based on previous experience with bevacizumab and current therapies, there is interest in exploring a potentially synergistic combination that targets both VEGF and immune checkpoint pathways for the treatment of advanced/metastatic TNBC. In addition, a phase III study of first-line PM8002/BNT327 plus nab-paclitaxel in locally inoperable advanced/metastatic TNBC is underway (NCT06419621), which will help clarify what PM8002/BNT327 may add to the standard of care,” she says. However, in developing these agents further, phase II randomised studies will be essential to understand if there is a strong signal that these add to the current standard of care with pembrolizumab and chemotherapy.
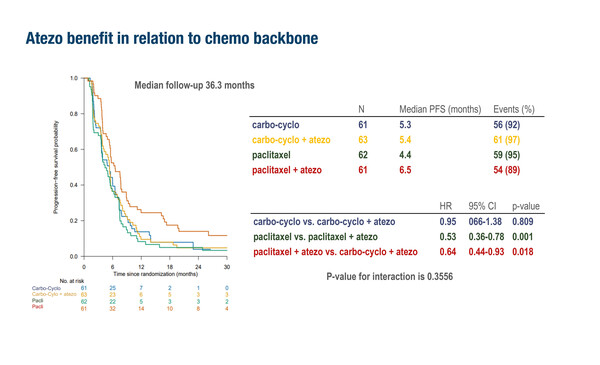
Focusing on optimising current therapy, investigators from the Netherlands presented further results of the Triple-B study, seeking to determine the most effective chemotherapy backbone for PD-L1 blockade (LBA20). They compared two different chemotherapy backbones (carboplatin–cyclophosphamide or paclitaxel) either with or without the addition of atezolizumab in patients with metastatic TNBC in a multicentre, open-label phase IIb trial using a 2×2 factorial design. Patients (N=305) were randomised first-line regardless of PD-L1 expression. The trial (NCT01898117) was started in 2013 and initially used bevacizumab (n=58) but changed to use atezolizumab (n=247) from January 2018 onwards. After a median follow-up of 36.3 months, paclitaxel plus atezolizumab was associated with the greatest improvement in median PFS (6.5 months) and higher ORR (54%) compared with carboplatin–cyclophosphamide with (PFS 5.4 months; ORR 38%) or without atezolizumab (PFS 5.3 months; ORR 31%) or paclitaxel alone (PFS 4.4 months; ORR 42%). For the 128 patients with confirmed homologous recombination deficiency (HRD), median PFS was prolonged the most in the carboplatin–cyclophosphamide plus bevacizumab/atezolizumab arm (n=31; 7.1 months), followed by carboplatin–cyclophosphamide (n=30; 5.8 months), paclitaxel plus bevacizumab/atezolizumab (n=29; 5.7 months) and paclitaxel (n=38; 3.9 months). The interaction between chemotherapy backbone (carboplatin–cyclophosphamide versus paclitaxel) and HRD status was not significant (interaction p-value of 0.1553). However, for the 101 patients with non-HRD tumours, there was a favourable PFS outcome (7.6 months) with first-line paclitaxel plus bevacizumab/atezolizumab compared with the other treatments (PFS 3.7 months – 5.7 months). There were 57 patients with PD-L1 CPS ≥10 and the median PFS with paclitaxel plus atezolizumab (n=18) was 7.6 months compared with 5.5 months with paclitaxel alone (n=18) in this subgroup (hazard ratio [HR] 0.37; 95% confidence interval [CI] 0.17–0.77; p=0.006), although no overall survival benefit was observed for the combination over paclitaxel alone (HR 0.87; 95% CI 0.37–2.01; p=0.742). The median PFS in those with PD-L1 CPS ≥10 who received carboplatin–cyclophosphamide (n=11) was 5.6 months, and 4.6 months in those (n=10) who received carboplatin–cyclophosphamide plus atezolizumab. “Although patient numbers in these analyses are small, overall, these results suggest that a paclitaxel chemotherapy backbone is preferable for combination with atezolizumab in the first-line setting for TNBC,” comments Loi.
Loi thinks there is much work to be done for the development of more effective strategies for advanced or metastatic TNBC. “Furthermore, increasing use of pembrolizumab in the early-stage setting will change the biology of patients whose disease recurs. With this in mind, we may need to develop separate strategies in the future for patients with recurrent disease who have and have not received prior pembrolizumab. Ultimately the survival of patients with advanced TNBC is still very poor and we must continue to identify the most effective novel treatments – whether it is combined PD-1/PD-L1 plus VEGF-targeting agents, other bispecific antibodies or double immune checkpoint inhibitor combinations – to give our patients the best chance of prolonged survival with the disease. These results offer hope that new treatments for advanced TNBC may help drive further future improvements in clinical care,” she concludes.
Programme details
Kok M, et al. First-line carboplatin-cyclophosphamide (CC) versus paclitaxel (P) with or without atezolizumab (atezo) for metastatic triple negative breast cancer (mTNBC): Results from a multicenter, randomized phase IIb trial: the Triple-B Study (BOOG 2013-01). ESMO Congress 2024, LBA20
Proffered Paper Session – Breast cancer, metastatic, 13.09.2024, h. 16:00 – 17:30, Barcelona Auditorium – Hall 2
Wang X, et al. The safety and efficacy of ivonescimab in combination with chemotherapy as first-line (1L) treatment for triple-negative breast cancer (TNBC). ESMO Congress 2024, Abstract 347MO
Mini Oral Session 2 – Breast cancer, metastatic, 16.09.2024, h. 08:30 – 10:00, Barcelona Auditorium – Hall 2
Wu J, et al. A phase Ib/II study to assess the safety and efficacy of PM8002/BNT327 in combination with nab-paclitaxel for first-line treatment of locally advanced or metastatic triple-negative breast cancer. ESMO Congress 2024, Abstract 348MO
Mini Oral Session 2 – Breast cancer, metastatic, 16.09.2024, h. 08:30 – 10:00, Barcelona Auditorium – Hall 2