In the TROPION-Breast01 trial, adverse events of special interest were generally low grade, easily managed and did not compromise scheduled treatment
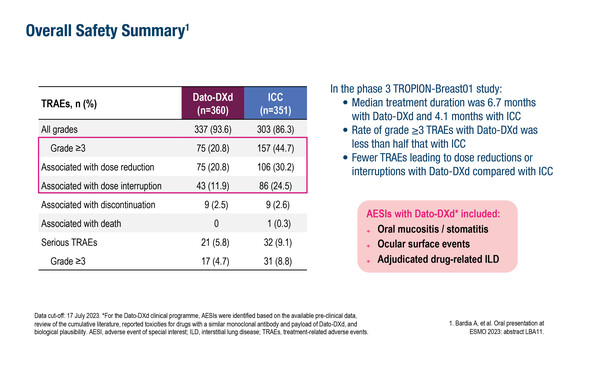
As reported at ESMO Breast Cancer 2024 (Berlin, 15–17 May), grade ≥3 treatment-related adverse events (TRAEs) were less common with the antibody–drug conjugate (ADC), datopotamab deruxtecan (Dato-DXd), versus investigator’s choice of chemotherapy (21% versus 45%) in 711 patients with previously treated inoperable or metastatic hormone receptor-positive (HR+)/HER2-negative (HER2−) breast cancer (LBA2). At the data cut-off of July 2023, the median treatment duration was longer with Dato-DXd compared with chemotherapy (6.7 months versus 4.1 months).
Given concerns regarding Dato-DXd adverse events of special interest – including stomatitis/oral mucositis and ocular toxicity – daily use of steroid-containing mouthwash was highly recommended and ophthalmological assessment was mandated as a regulatory requirement at screening and every three 21-day cycles. Stomatitis/oral mucositis (56%) and ocular surface events (40%) were generally low-grade (grade 1 in 25% and 32% of all Dato-DXd patients, respectively). Adjudicated drug-related interstitial lung disease (ILD) – another adverse event of special interest – was reported in 3% of patients receiving Dato-DXd and was mainly low grade. Adverse events of special interest generally occurred in the first few cycles of Dato-DXd, with the median onset of stomatitis/oral mucositis at cycle 2, ocular surface events at cycle 3 and ILD at cycle 4. The corresponding median times to resolution were 37, 67 and 28 days, respectively.
Dato-DXd-mediated stomatitis/oral mucositis and ocular surface toxicity were well managed with toxicity management guidelines, resulting in uncommon dose interruptions (1% and 3%, respectively) and discontinuations (0.3% each). For chemotherapy, high-grade neutropenia (grade ≥3 in 31%) was often associated with dose interruptions (17%) and reductions (13%).
ADCs are changing the shape of breast cancer treatment. These additional safety findings from the phase III TROPION-Breast01 trial further support the primary results – presented at the ESMO Congress 2023 (Ann Oncol. 2023;34:Suppl.2;S1264–S1265) – which showed the significant progression-free survival benefits of Dato-DXd over chemotherapy. Mature overall survival results are awaited.
Abstract discussed:
Jhaveri K, et al. Datopotamab deruxtecan (Dato-DXd) vs chemotherapy (CT) in pretreated, inoperable/metastatic HR+/HER2– breast cancer (BC): Additional safety analysis from TROPION-Breast01. ESMO Breast Cancer 2024, LBA2
Proffered Paper Session 2, 16.05.2024, h. 16:45 – 18:05, Berlin Hall