Results from the Neo-CheckRay study report an acceptable toxicity profile with the addition of a PD-L1 inhibitor to neoadjuvant chemotherapy and stereotactic body radiation therapy
Initial results from the phase II Neo-CheckRay study, presented at the ESMO Congress 2024 (Barcelona, 13–17 September), suggest a beneficial effect with the addition of durvalumab (± oleclumab) to neoadjuvant chemotherapy (NACT) and stereotactic body radiation therapy (SBRT) compared with NACT plus SBRT alone in patients with early-stage, high risk, oestrogen receptor (ER)-positive/HER2-negative breast cancer (LBA10).
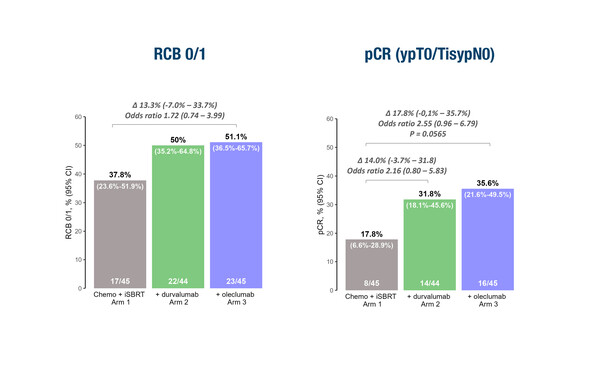
In this first prospective, randomised study to investigate this treatment combination, which involved 135 patients with evaluable data, the rates of residual cancer burden (RCB) 0–1 – the primary endpoint – and pathological complete response (pCR) at surgery were numerically increased following the addition of a PD-L1 inhibitor (durvalumab) and an adenosine-targeting agent (oleclumab) to NACT plus SBRT. RCB 0 (or pCR) was achieved by 35.6% of 45 patients who received durvalumab and oleclumab with NACT plus SBRT compared with 17.8% of the 45 patients who received NACT and SBRT alone.
RCB score is independently prognostic for event-free survival (EFS) in all subtypes of breast cancer; reduced EFS is reported in patients with tumours that do not achieve a pCR (or RCB 0) after NACT (Lancet Oncol. 2022;23:149–160).
Commenting on the Neo-CheckRay study design, Prof. Charlotte Coles from the University of Cambridge, UK, explains that neoadjuvant immunoradiation is an innovative strategy in this setting as the standard of care is to give a tumour bed boost at the same time or after whole breast radiotherapy (RT) for patients with tumours at high risk of local recurrence. In the study, the immunoradiation boost is brought forward to the pre-operative setting, providing an immune primer to the tumour, while still allowing routine whole-breast RT to be given following surgery.
“The immunoradiation boost may convert an immunologically cold tumour into an inflamed tumour and could also have effects on distant non-irradiated sites through the abscopal effect, a systemic immune response observed with immune modulators in some situations, but rarely with RT alone,” notes Coles. “By excising the irradiated tumour at surgery, late fibrosis in the breast may also be limited and the effect on breast cosmesis minimised.”
Early toxicity appeared to be acceptable. While patients in the experimental arms (Arm 2: NACT plus SBRT plus durvalumab; Arm 3: Arm 2 plus oleclumab) experienced a higher incidence of treatment-related and immune-related adverse events (AEs) than those in Arm 1 (NACT plus SBRT), serious AEs occurred with a similar incidence across all three arms (10.4%, 17.6% and 16.7%, respectively). “It will be important to see longer-term data relating to toxicity, especially given the high dose of RT (3 x 8 Gy) used for the tumour bed boost,” Coles cautions.
She describes the results as “exciting”, although notes that, “all study arms included SBRT, so we cannot be sure that the effect on RCB required the addition of RT.” Coles considers the innovative study design to be an ideal platform to investigate novel RT–drug combinations in the neoadjuvant setting for high-risk disease. “However, it will be important to see additional outcomes, such as invasive disease-free survival 3 years after surgery, and to determine the optimal dose fractionation for immune priming that has acceptable toxicity,” she concludes. “The study showed that a positive collaboration between radiation oncologists and medical oncologists is an excellent framework to build on in the future, which could lead to a better understanding of biomarkers for improved patient selection.”
Programme details
De Caluwe A, et al. Primary endpoint results of the Neo-CheckRay phase II trial evaluating stereotactic body radiation therapy (SBRT) +/- durvalumab (durva) +/- oleclumab (ole) combined with neo-adjuvant chemotherapy (NACT) for early-stage, high risk ER+/HER2- breast cancer (BC). ESMO Congress 2024, LBA10
Proffered Paper Session – Breast cancer, early stage, 16.09.2024, h. 10:15 – 11:45, Barcelona Auditorium – Hall 2