Final results from the KEYNOTE-522 study confirm that the integration of pembrolizumab into the treatment regimen has the potential to transform patient management, but careful patient selection and monitoring for toxicities will be crucial
“The final overall survival (OS) data from the KEYNOTE-522 study represent some of the most significant results observed in the treatment of early breast cancer,” says Dr Carmen Criscitiello from the European Institute of Oncology, Milan, Italy, commenting on data reported in a Presidential Symposium at the ESMO Congress 2024 (Barcelona, 13–17 September; LBA4)
The phase III KEYNOTE-522 study previously showed statistically significant and clinically meaningful improvements in the co-primary endpoints pathological complete response (pCR) (N Engl J Med. 2020;382:810–821) and event-free survival (EFS) (N Engl J Med. 2022;386:556–567) with the addition of pembrolizumab to standard chemotherapy in 1,174 patients with early-stage triple-negative breast cancer (TNBC).
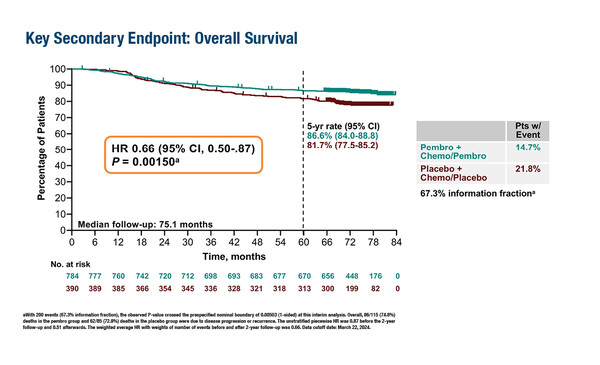
In the final analysis, at a median follow-up of 75.1 months, 115 of 784 (14.7%) patients randomised to the pembrolizumab arm had died compared with 85 of 390 (21.8%) patients randomised to the control arm, and the pre-specified significance boundary of 0.00503 was met (hazard ratio [HR] 0.66; 95% confidence interval [CI] 0.50–0.87; p=0.0015).
The respective 5-year OS rates were 86.6% versus 81.7%, while the 5-year EFS rate was 81.2% in the pembrolizumab arm versus 72.2% in the placebo arm (HR 0.65; 95% CI 0.51–0.83). “The results confirm the potential of pembrolizumab as a key component of combination treatment regimens for high-risk, early-stage TNBC, and pembrolizumab added to chemotherapy should be considered a new standard of care in this setting,” notes Criscitiello. “The data are particularly relevant as TNBC is a subtype associated with poor prognosis and few treatment options. The integration of immunotherapy into earlier stages of treatment has the potential to transform the management of these patients.”
Reflecting on key data from the KEYNOTE-522 study and how these might influence clinical practice, Criscitiello points out: “The 5-year EFS was 72.2% in the placebo arm, showing that many patients are doing well with chemotherapy alone. We therefore need to be able to identify which patients are likely to have a favourable prognosis without immunotherapy, so that we avoid immune-related toxicity in these patients.” Immune-mediated adverse events of any grade were reported in 35.0% of patients receiving pembrolizumab versus 13.1% of those receiving placebo. This is an important consideration in the pre-operative setting as it may result in a delay to surgery, which could compromise patient outcomes. “In addition, there are financial considerations of incorporating pembrolizumab into treatment regimens as many countries have restrictions and are not able to reimburse the immunotherapy. While the benefits of having pembrolizumab are significant, consideration of these factors will be crucial for the effective and responsible integration of the KEYNOTE-522 regimen into clinical practice,” she says.
The study reported grade ≥3 treatment-related adverse event rates of 77.1% in the pembrolizumab arm and 73.3% in the placebo arm. “Monitoring of longer-term safety will be essential, and understanding the balance between potential survival benefit and risk of toxicities will be crucial in determining the overall long-term value of this regimen,” adds Criscitiello.
Given the toxicity associated with combined chemotherapy plus immunotherapy regimens, an exploratory study also presented at the Congress compared two cohorts (15 patients in each), with TNBC and high tumour-infiltrating lymphocytes (TILs) who received short-term chemotherapy-free neoadjuvant therapy; combination immunotherapy – either nivolumab plus ipilimumab or nivolumab plus the anti-LAG3 agent relatlimab – was administered prior to surgery (LBA11). The pCR rate (primary endpoint) was 33% with nivolumab plus ipilimumab and 47% with nivolumab plus relatlimab. Overall, 7 (23.3%) patients developed grade 3–4 adverse events (6 of whom received nivolumab plus ipilimumab). “In this study, the efficacy data are particularly promising given the absence of chemotherapy and provided the toxicity is manageable, especially in patients treated with the nivolumab plus ipilimumab combination,” comments Criscitiello.
Both studies underline the need for careful patient selection and monitoring, particularly when considering treatment toxicities and Criscitiello thinks it will be essential to further refine these treatment strategies, potentially moving towards a less toxic chemotherapy regimen for early-stage TNBC in the future. “We also need to continue our exploration of different immunotherapy combinations – including combinations with other novel agents – to maximise efficacy and minimise toxicity.” Other areas of interest are biomarker research to enable more accurate identification of patients who are most likely to benefit from immunotherapy, monitoring of late-onset toxicities, particularly in patients who receive chemotherapy-free regimens, and exploration of personalised approaches based on patient and tumour characteristics, and potentially also genomic and immune profiling.
Programme details
Schmid P, et al. Neoadjuvant pembrolizumab or placebo plus chemotherapy followed by adjuvant pembrolizumab or placebo for high-risk early-stage TNBC: Overall survival results from the phase 3 KEYNOTE-522 study. ESMO Congress 2024, LBA4
Presidential Symposium II – Practice-changing trials, 15.09.2024, h. 16:30 – 17:50, Barcelona Auditorium – Hall 2
Nederlof I, et al. Neoadjuvant nivolumab/relatlimab or nivolumab/ipilimumab in triple negative breast cancer with high tumor-infiltrating lymphocytes (TILs). ESMO Congress 2024, LBA11
Proffered Paper Session – Breast cancer, early stage, 16.09.2024, h. 10:15 – 11:45, Barcelona Auditorium – Hall 2