The combination therapy with the CDK 4/6 inhibitor improved overall response rate compared to standard treatment
Results from the ABIGAIL study showed that the CDK 4/6 inhibitor abemaciclib combined with endocrine therapy (ET) achieved a higher early overall response rate (ORR) at 12 weeks compared to standard chemotherapy as first-line treatment in patients with hormone receptor–positive (HR+)/HER2-negative advanced breast cancer with aggressive disease characteristics. Data were presented at the ESMO Congress 2024, just ended in Barcelona, Spain.
CDK4/6 inhibitors plus ET represent one of the standard first-line therapies for HR+/HER2- advanced breast cancer, however chemotherapy as induction treatment is still used for patients at risk of rapid progression.
In the ABIGAIL trial – an open-label, multicenter, randomised phase 2, non-inferiority study – 162 patients with HR+/HER2- advanced breast cancer were randomised to either receive oral abemaciclib (150 mg twice/day in 28-day cycles) plus oral letrozole (2.5 mg/day) or fulvestrant via intramuscular injection (500 mg/days 1, 15, 29 and once monthly thereafter) at investigator criteria, or intravenous paclitaxel (90 mg/m2, days 1, 8, and 15 every 28 days) for 12 weeks followed by abemaciclib plus ET (letrozole or fulvestrant at investigator criteria). All patients had not received prior systemic therapy for their advanced disease, and presented at least one of the following criteria for aggressive disease: visceral metastases, grade 3 and/or negative progesterone receptor on primary tumor, LDH >1.5 x ULN, and/or disease progression on or within 36 months after completing adjuvant ET.
The study met its primary end point in the intent-to-treat population with a 12 week-ORR of 58.8% in patients treated with abemaciclib plus ET compared to 40.2% in patients treated with chemotherapy (OR: 2.12 [95% CI, 1.13–3.96]; p=0.019).
“A key question is whether induction chemotherapy is needed for some patients. We will have to wait for the progression-free survival (PFS) data from the trial to be mature to answer this question”, stated Dr Sandra Ximena Franco Millan, Country Clinic, Bogota, Colombia, discussing the study findings at the Mini-Oral session. “Currently, we do know that 15-20% of patients have rapid progression while on therapy with CDK 4-6 inhibitors, but we just do not know who they are: they may have different mutations such as PI3K or AKT, or maybe a heterogeneity of the disease with some cells that are not really endocrine-sensitive. A short course of chemotherapy may benefit them.”
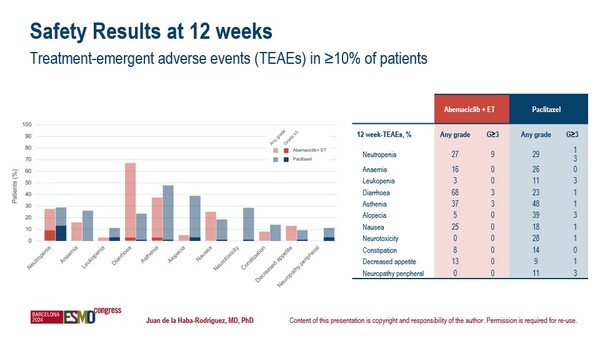
Safety data presented at the Congress were in line with the toxicity profiles of each treatment strategy, with any treatment-emergent adverse events (TEAEs) observed in ≥10% of patients including neutropenia (27% vs 29%), anaemia (16% vs 26%), diarrhoea (68% vs 23%), asthenia (37% vs 48%) and nausea (25% vs 18%) (Figure).
Abstract discussed at the ESMO Congress 2024
Juan De la Haba Rodriguez et al. ABIGAIL: Randomized phase II study of abemaciclib plus endocrine therapy (ET) with or without a short course of induction paclitaxel in patients (pts) with previously untreated HR-positive/HER2-negative advanced breast cancer (HR+/HER2- ABC) with aggressive disease criteria. ESMO Congress 2024, LBA23