Analyses of FLAURA2, MARIPOSA and MARIPOSA-2 trials provide further guidance on treatment choices for advanced lung cancer, but questions remain about optimal sequencing
Positive results from two analyses of post-progression outcomes presented at the European Lung Cancer Congress 2024 (Prague, Czech Republic, 20–23 March) help to confirm the prolonged benefits of osimertinib- and amivantamab-based combinations for patients with EGFR-mutated advanced non-small cell lung cancer (NSCLC).
In a first report, from the FLAURA2 trial, which previously demonstrated an improvement in progression-free survival (PFS) with first-line osimertinib plus chemotherapy (N Engl J Med. 2023;389:1935–1948), the combination was associated with post-progression benefits over osimertinib alone in the time to the first subsequent therapy (30.7 months versus 25.4 months; hazard ratio [HR] 0.73), second progression-free survival (PFS2) (HR 0.70) and time to second subsequent therapy (HR 0.69) (Abstract 4O).
A second report of post-progression outcomes, from the MARIPOSA-2 trial in patients progressing on osimertinib, showed that the combination of amivantamab plus chemotherapy significantly prolonged time to treatment discontinuation (11.0 months versus 4.5 months; HR 0.37), time to subsequent therapy (HR 0.42) and PFS2 (HR 0.60) compared with chemotherapy alone (Abstract 3MO).
“The delay in time to subsequent therapy and the improvement in second PFS observed in both the FLAURA2 and MARIPOSA-2 trials are very encouraging in terms of highlighting positive effects beyond the initial PFS,” says Prof. Zofia Piotrowska from the Massachusetts General Hospital/Harvard Medical School, Boston, MA, USA. “In addition, nearly two-thirds of patients in the MARIPOSA-2 trial were able to receive subsequent therapy, which is important when you are starting to look at third-line therapy for this cohort.”
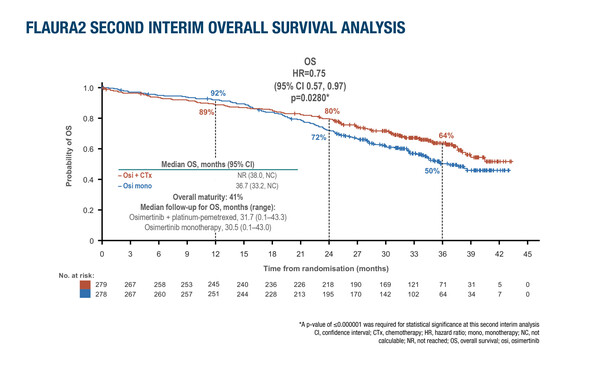
Also, a second interim analysis of overall survival (OS) data from the FLAURA2 trial, at around 30 months’ follow-up, showed a trend towards benefit with the osimertinib combination (HR 0.75; 95% confidence interval 0.57–0.97; p=0.0280), which did not reach the prespecified level of statistical significance (p≤0.000001). Although the data are not conclusive, according to Piotrowska, the trend in the longer follow-up is a signal that “we are travelling in the right direction.”
Efficacy with these new combinations appears to be heralding a new era in treatment; however, caution is needed not to neglect the potential impact of adverse events on therapy. Data from MARIPOSA-2 report that haematological toxicities in the first cycle were more common with amivantamab plus chemotherapy compared with chemotherapy, although by cycle 2, the incidence was similar between treatment arms. Of note, a trend towards an improvement in time to symptomatic progression with amivantamab plus chemotherapy versus chemotherapy (median 14.9 versus 13.0 months; HR 0.74; p=0.10) was shown when patient-reported outcomes (PROs) were analysed from MARIPOSA-2 (Abstract 8P). Amivantamab plus chemotherapy was also associated with benefits in terms of improved or stable physical functioning, emotional functioning, cognitive functioning and global health status, as well as no dyspnoea, no pain and no fatigue. “PROs are really important in helping us to understand if the side effects in trials relate to patients’ experience of treatment. And it is encouraging to see that in the MARIPOSA-2 trial, the amivantamab plus chemotherapy combination resulted in stable, and even improved, perceptions of treatment,” Piotrowska observes.
Also presented at the ELCC 2024, results from an exploratory analysis of the MARIPOSA trial revealed that early interruptions of first-line amivantamab plus lazertinib due to adverse events did not negatively impact outcomes (Abstract 5MO). Nearly half of patients receiving the combination treatment required amivantamab dose interruptions by 4 months. At a median follow-up of 22.0 months, the median PFS was 27.5 months for patients with dose interruptions during the first 4 months of treatment compared with 25.7 months for all those receiving amivantamab plus lazertinib.
“Altogether, these updated data highlight the benefits of new combinations, but going forward, we need to better define optimal sequencing for different patient cohorts,” says Piotrowska underlining the key role biomarkers will play in identifying patients most likely to benefit. “By using a dynamic biomarker, such as circulating tumour DNA, we can identify patients at high risk of progression following initiation of a new treatment and then tailor subsequent treatments,” she concludes.
Abstracts dicussed:
Valdiviezo Lama NI, et al. First-line (1L) osimertinib (osi) ± platinum-pemetrexed in EGFR-mutated (EGFRm) advanced NSCLC: FLAURA2 post-progression outcomes. European Lung Cancer Congress 2024, Abstract 4O
Proffered Paper Session, 21.03.2024, h. 15:10 – 16:40, Congress Hall
Gentzler RD, et al. Amivantamab plus chemotherapy vs chemotherapy in EGFR-mutant advanced NSCLC after progression on osimertinib: A post-progression analysis of MARIPOSA-2. European Lung Cancer Congress 2024, Abstract 3MO
Mini Oral Session 2, 21.03.2024, h. 17:00 – 17:50, Forum Hall
García Campelo MR, et al. Effect of amivantamab dose interruptions on efficacy and safety of first-line amivantamab plus lazertinib in EGFR-mutant advanced NSCLC: Exploratory analyses from the MARIPOSA study. European Lung Cancer Congress 2024, Abstract 5MO
Mini Oral Session 2, 21.03.2024, h. 17:00 – 17:50, Forum Hall
Tomasini P, et al. Amivantamab plus chemotherapy vs chemotherapy in EGFR-mutant advanced NSCLC after progression on osimertinib: Secondary analyses of patient-relevant endpoints from MARIPOSA-2. European Lung Cancer Congress 2024, Abstract 8P
Poster Display Session, 22.03.2024, h. 12:00 – 12:45, Congress Hall Foyer