Patients consider side-effects of the KRAS G12C inhibitor to be more tolerable and to have less interference on their lives than those due to docetaxel
In a presentation at the European Lung Cancer Congress 2023 (Copenhagen, 29 March–1 April), quality of life (QoL) results from the phase III CodeBreaK 200 trial demonstrated that patients with KRAS G12C-mutated non-small cell lung cancer (NSCLC) who progressed after receiving platinum-based chemotherapy and a checkpoint inhibitor experienced more bothersome side-effects (odds ratio [OR] 5.71) with a negative impact on patients’ usual/daily activities when treated with docetaxel compared with sotorasib (Abstract 4O). Patients on docetaxel experienced symptoms that were more severe, including pain (OR 2.94), aching muscles (OR 4.40), aching joints (OR 4.17) and mouth or throat sores (OR 4.26; all p<0.05).
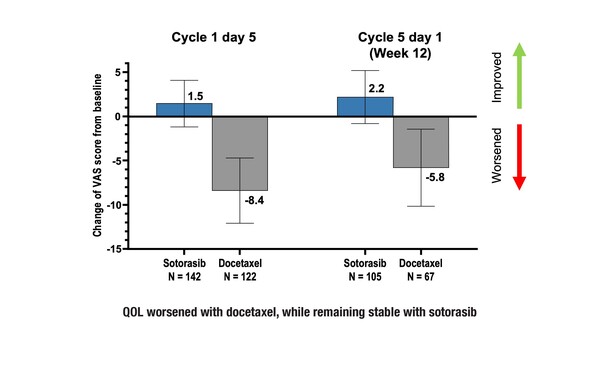
EuroQol-5 dimension visual analogue scale (EQ-5D VAS) scores were comparable between the arms at baseline, being 67.6 for sotorasib and 68.3 for docetaxel. However, five days after initial treatment, QoL worsened in patients receiving docetaxel but remained stable in those receiving sotorasib, with EQ-5D VAS score changes from baseline of –8.4 and 1.5, respectively. This pattern was maintained at week 12, with EQ-5D VAS score changes of –5.8 for docetaxel and 2.2 for sotorasib.
“QoL is a really important endpoint in the setting of patients receiving second-line treatment for lung cancer, who have limited life expectancy and whose overall condition after the failure of first-line treatment can be quite poor,” explains Prof. Massimo Di Maio from the University of Turin at Ordine Mauriziano Hospital, Italy. “We know from the initial analysis of the CodeBreaK 200 trial that sotorasib significantly prolonged progression-free survival compared with docetaxel, although the benefit was quite small (Lancet. 2023;401:733–746), and there was no overall survival advantage. In this type of situation, instrumental control alone (especially if quite modest in magnitude) does not necessarily imply clinical benefit for the patient, and patient-reported outcomes (PROs) are particularly important in helping to determine the value of one treatment over another,” he says, highlighting that limited efficacy of docetaxel comes with greater negative impact on patients’ health status in terms of fatigue and functional status.
“While the data presented are interesting and help to confirm PRO findings reported in the original publication, in fact, their clinical relevance may be relatively modest. QoL results in cases like this are not only conditioned by the efficacy in improving the symptoms of the disease, but also by the burden of side-effects associated with the treatment. From this point of view, unfortunately, being superior to standard 3-weekly docetaxel in terms of QoL does not necessarily mean an optimal outcome,” concludes Di Maio. “Other targeted agents such as EGFR inhibitors or ALK inhibitors have been shown to have a greater and more substantial impact on clinical outcomes, including QoL, versus chemotherapy.”
Abstract presented:
Waterhouse DM, et al. Patient-reported outcomes from the CodeBreaK 200 phase 3 trial comparing sotorasib versus docetaxel in KRAS G12C-mutated NSCLC. European Lung Cancer Congress 2023, Abstract 4O
Proffered Paper 1, 29.03.2023, h. 16:30 – 18:00, Auditorium 1