Results from the CodeBreaK 200 trial represent an improvement for a difficult-to-treat patient population
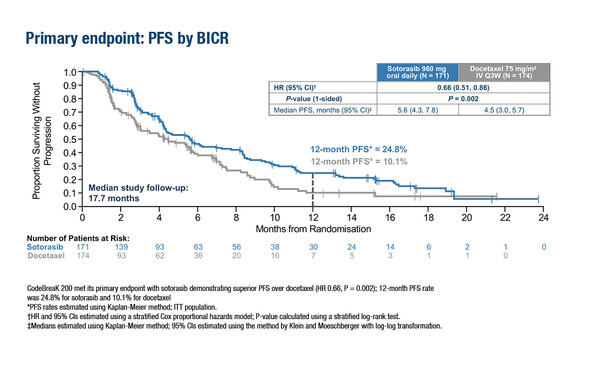
The primary analysis of the phase III CodeBreaK 200 trial in patients with KRAS G12C-mutated non-small-cell lung cancer (NSCLC) who had progressed after prior platinum-based chemotherapy and a checkpoint inhibitor showed the study met its primary endpoint of a statistically significant improvement in progression-free survival (PFS) with sotorasib versus docetaxel (hazard ratio 0.66; 95% confidence interval [CI] 0.51–0.86; p=0.002) after a median follow up of 17.7 months (LBA10). One-year PFS rates were 24.8% for sotorasib and 10.1% for docetaxel and PFS benefit was consistent across subgroups. The objective response rate (ORR) was also significantly higher with sotorasib (28.1%; 95% CI 21.5–35.4%) than with docetaxel (13.2%; 95% CI 8.6–19.2%; p<0.001); disease control rate was 82.5% versus 60.3%, respectively. Overall survival (OS) did not differ between groups although the study was not powered for OS. Sotorasib also had a more favourable safety profile than docetaxel.
“These results represent an advancement for the treatment of patients with KRAS G12C-mutated NSCLC, and confirm the role of KRAS G12C inhibitors for pre-treated patients with advanced/metastatic disease harbouring KRAS G12C mutations,” says Dr Antonio Passaro, from the European Institute of Oncology, Milan, Italy, commenting on the study findings. “Sotorasib previously received accelerated US FDA and conditional EMA approval in the same setting based on ORR data from the single-arm phase I/II CodeBreaK 100 trial (Clin Cancer Res. 2022;28:1482–1486; Amgen press release), but a comparative evaluation was needed to improve the understanding of this molecular-driven disease and move forward the approval in countries where sotorasib is not yet reimbursed.”
Passaro cautions against comparing these data for a KRAS G12C inhibitor with those using EGFR or ALK inhibitors, noting that, “KRAS G12C mutation is a confirmed novel therapeutic target in NSCLC and the KRAS G12C covalent inhibitors, that lock the GTPase protein in the inactive KRas-GDP state, showed a different mechanism of action to the well-known TKIs that target mutations or alterations on genes encoding receptor tyrosine kinases, such as EGFR or ALK” continues Passaro.
“The PFS and ORR data for sotorasib are not impressive and are of modest impact, but for patients with advanced/metastatic NSCLC who have already received one or two therapeutic treatments, the option of receiving an oral agent with a superior PFS and ORR and a more favourable safety profile, compared with standard of care intravenous docetaxel, is a significant and clinically relevant improvement.” On the lack of OS benefit, Passaro says, “While the OS results are important in a trial of second-line therapy, they were not significant, although the study was not powered for OS and crossover was permitted following disease progression, thereby limiting insights into this important endpoint.”
“These results, while supporting a new treatment option, confirm that further improvements are highly sought after for this specific molecular-driven patient population, and different kinds of combinations are under evaluation in both treatment-naïve and pre-treated settings (N Engl J Med 2022; 387:180–183),” Passaro concludes.
Johnson ML, et al. Sotorasib versus docetaxel for previously treated non-small cell lung cancer with KRAS G12C mutation: CodeBreaK 200 phase III study. ESMO Congress 2022, LBA10
Presidential Symposium III, 12.09.2022, h. 16:30 – 18:15, Paris Auditorium