Preliminary results revealed that the combination of claudin 18.2/4-1BB bispecific antibody with nivolumab and mFOLFOX was also well tolerated
As reported at the ESMO Gastrointestinal Cancers Congress 2025 (Barcelona, 2–5 July), treatment with the claudin (CLDN) 18.2/4-1BB bispecific antibody, givastomig, showed an objective response rate of 71% when combined with nivolumab and mFOLFOX in the dose-escalation period of a phase Ib trial in patients with HER2-negative, CLDN18.2-positive gastric, oesophageal or gastro-oesophageal adenocarcinomas (Abstract 388MO). CLDN18.2 positivity was defined as ≥1+ intensity in ≥1% of tumour cells detected by immunohistochemistry (IHC).
All responses with givastomig combination therapy were partial and were seen in 2 out of 5 patients at 5 mg/kg, 5 out of 6 patients at 8 mg/kg and 5 out of 6 patients at 12 mg/kg. The disease control rate was 100%. Responses occurred irrespective of PD-L1 combined positive score (CPS≥5 or <5) or CLDN18.2 expression (≥75% or <75%). Eight patients are continuing in the study. The 6-month progression-free survival rate was 72.9% across all three doses studied and 81.5% in patients who received 8 mg/kg or 12 mg/kg.
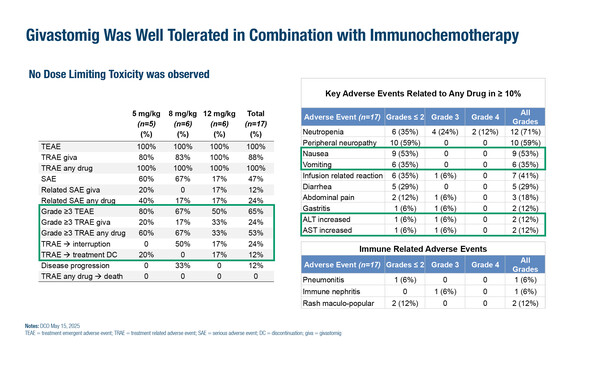
No dose-limiting toxicities (DLTs) were observed and the maximum tolerated dose (MTD) was not reached. Treatment-related adverse events (TRAEs) included neutropenia (71%), peripheral neuropathy (59%), nausea (53%), infusion-related reactions (IRRs) (41%) and vomiting (35%). Enrollment of the 8 mg/kg dose-expansion cohort is now complete (n=20), while enrollment at 12 mg/kg is ongoing.
“This novel concept of a triple combination reaches beyond what has been established so far. Although this study included a small patient population, the response and disease control rates are outstanding and, together with a favourable safety profile, the findings support the further investigation of this treatment,” observes Prof. Thomas Seufferlein from the University of Ulm, Germany. He points out that the approach used for CLDN18.2 positivity differs from that used in trials for the recently approved monoclonal antibody, zolbetuximab, where the definition was moderate-to-strong membranous CLDN18.2 IHC staining in ≥75% of tumour cells (Lancet. 2023;401:1655–1668; Nat Med. 2023;29:2133–2141). “In the current study, the definition of ≥1+ intensity in ≥1% of tumour cells reduces the requirement for a positive signal. And the researchers were able to show that responses to givastomig were seen in patients with <75% CLDN18.2 who would not normally be eligible for anti-CLDN18.2 treatment,” he explains.
Seufferlein concludes: “The usefulness of CLDN18.2 as a target has attracted investigation of a number of novel therapies, including bispecific antibodies, CAR T-cells and antibody–drug conjugates. Combining different approaches that might be acting synergistically is an exciting and promising avenue of research.”
Programme details
Klempner SJ, et al. Preliminary safety and efficacy of givastomig, a novel claudin 18.2/4-1BB bispecific antibody, in combination with nivolumab and mFOLFOX in metastatic gastroesophageal carcinoma (mGEC). ESMO Gastrointestinal Cancers Congress 2025, Abstract 388MO
Mini Oral Session – Innovation in GI Cancers, 02.07.2025, h. 16:15 – 17:35, Room Barcelona