Randomised trials and real-world data propel the anti-EGFR rechallenge approach towards standard of care, but more solid evidence is needed
Data from the PARERE and OrigAMI-1 trials, as presented at the ESMO Gastrointestinal Cancers Congress 2025 (Barcelona, 2–5 July), provide evidence that circulating tumour (ct) DNA-guided anti-EGFR rechallenge, by different inhibitory approaches, is an effective strategy in RAS/BRAF wild-type metastatic colorectal cancer (mCRC).
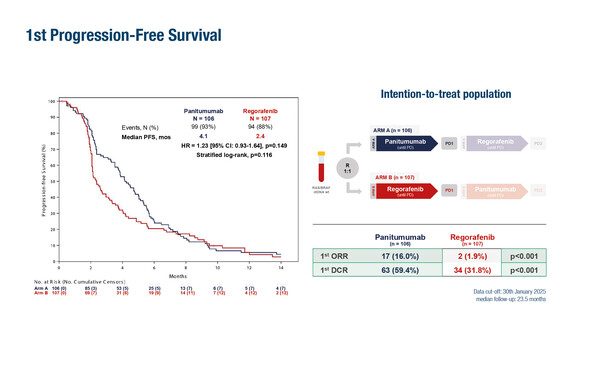
In the phase II PARERE trial, a sequence of EGFR inhibition with panitumumab followed by regorafenib at progression was associated with better outcomes than the reverse sequence among 213 patients with chemorefractory tumours, who had responded to first-line anti-EGFR treatment (Abstract 4O). Panitumumab led to a higher objective response rate (ORR) (16% versus 2%; p<0.001) and disease control rate (DCR) (59% versus 32%; p<0.001) than regorafenib. Progression-free survival 1 (PFS1) was 4.1 months with panitumumab and 2.4 months with regorafenib (stratified log-rank p=0.116). The PFS advantage of panitumumab was more evident among ‘hyper-selected’ patients with ctDNA RAS/BRAF wild-type and there was no difference between panitumumab and regorafenib among 19 patients who had at least one anti-EGFR resistance mutation.
Updated results from the phase Ib/II OrigAMI-1 trial demonstrated that a longer interval (>8.8 months versus ≤8.8 months) before rechallenge with the EGFR-MET bispecific antibody amivantamab led to an improved ORR (32% versus 7%) and median PFS (7.0 months versus 2.8 months; nominal p=0.0081), with a numerical trend towards a longer overall survival (OS) (16.1 months versus 10.4 months; nominal p=0.088), in 54 patients with left-sided ctDNA RAS/BRAF wild-type mCRC (Abstract 6MO). Baseline ctDNA mutational analysis was not predictive of response.
“The response rates achieved in both trials are meaningful in the highly pre-treated setting and the DCR in the PARERE trial is extremely encouraging,” says Prof. Jenny Seligmann from the University of Leeds, UK. "The results confirm, in larger, randomised settings, the findings from the previously reported, small, single-arm CHRONOS study (Nat Med. 2022;28:1612–1618).”
Also presented at the Congress, a poster reported real-world outcomes from a retrospective Spanish study of 134 patients with RAS/BRAF wild-type mCRC and indicated that a longer prior anti-EGFR PFS (>20 months) and anti-EGFR treatment-free interval (>10.4 months) were each associated with a better DCR (p=0.04 and p<0.01, respectively) and PFS (p=0.01 and p<0.01) – and, for the EGFR-free interval, OS (p<0.01) – compared with shorter intervals (Abstract 42P). Seligman thinks investigating the ideal interval between treatments is important and is reassured that rechallenge effects extend to patients treated in the clinic, although the cohort was treated at a highly specialised, expert centre.
She concludes by observing that this is currently a very active area of research and new data “propel the anti-EGFR rechallenge approach towards a standard of care.” However, she highlights that additional studies and considerations are needed: “Challenges ahead include formally defining both the timing of anti-EGFR rechallenge within the treatment pathway and the timing of ctDNA in directing rechallenge. Also, as exciting as these data are, we should remember that liquid biopsy-directed treatment is likely to have restricted patient availability, given that many centres do not have access to the necessary ctDNA technology.”
Programme details
Ciracì P, et al. Liquid biopsy-guided selection for anti-EGFR re-treatment in RAS/BRAF wild-type (wt) chemorefractory metastatic colorectal cancer patients (mCRC pts): Results from the phase II randomized PARERE trial. ESMO Gastrointestinal Cancers Congress 2025, Abstract 4O
Proffered Paper Session 2, 04.07.2025, h. 16:30 – 18:00, Room Madrid
Arnold D, et al. Rechallenge with amivantamab, an EGFR-MET bispecific antibody, after disease progression on prior EGFR inhibitor in left-sided RAS/BRAF wild-type metastatic colorectal cancer: Updated results from OrigAMI-1. ESMO Gastrointestinal Cancers Congress 2025, Abstract 6MO
Mini Oral Session – Innovation in Gl Cancers, 02.07.2025, h. 16:15 – 17:35, Room Barcelona
Mascaró Baselga P, et al. Real-world outcomes of anti-EGFR rechallenge in RAS/BRAF wild-type (wt) metastatic colorectal cancer (mCRC). ESMO Gastrointestinal Cancers Congress 2025, Abstract 42P
Poster Display Session 2, 04.07.2025, h. 15:30 – 16:30, Foyer