Quality of life and pain management data adds to efficacy results of combining TTFields with gemcitabine and nab-paclitaxel as a potential new option of care
Tumor Treating Fields (TTFields), a non-invasive electric-based therapy that disrupts cancer cells’ ability to proliferate, showed to improve Quality of Life (QoL) and pain management in patients with unresectable locally advanced pancreatic adenocarcinoma (LAPC), according to results from the PANOVA-3 presented at the ESMO Gastrointestinal Cancers Congress 2025 earlier this month (LBA3). These results followed on a prior presentation at the 2025 ASCO Annual Meeting, where the treatment had shown to impact on overall survival (OS) in this patient population (J Clin Oncol 2025, 43, LBA4005-LBA4005).
Pain affects approximately 80% of patients with pancreatic cancer and is associated with reduced survival (Dig Dis Sci. 2017 Apr;62(4):861-870). Therefore, pain management represents a critical issue which often requires the use of morphine-based analgesics. Investigators of the global phase III PANOVA-3 trial recently reported significantly prolonged overall survival (OS), pain-free survival, and progression-free survival benefits when TTFields was combined with gemcitabine/nab-paclitaxel compared to gemcitabine/nab-paclitaxel alone in 571 patients with newly diagnosed LAPAC, with no additive systemic toxicity (J Clin Oncol 0, JCO-25-00746).
In the study, 571 patients had been randomised to receive Gemcitabine and NabPaclitaxel (GnP) with or without TTFields. The study met its primary endpoint, with a significantly longer OS with TTFields/GnP than with GnP - respectively 16.2 (95% CI 15.0-18.0) versus 14.2 months (95% CI 12.8-15.4) (hazard ratio (HR) 0.82; 95% CI 0.68-0.99;, p=0.039). In contrast, there was no significant difference in progression-free survival (PFS) - median 10.6 (95% CI 9.2-12.2) vs 9.3 months (95% CI 7.6-11.1); (HR 0.85; 95% CI 0.68-1.05, p=0.137) - and ORR was similar between arms - 36.1% (95% CI 30.0-42.4) vs 30.0% (95% CI 24.3-36.2) (p=0.094). Very interestingly, pain-free survival was significantly longer with TTFields/GnP compared to GnP, respectively with a median of 15.2 (95% CI 10.3-22.8) vs 9.1 months (95% CI 7.4-12.7); HR 0.74 (95% CI 0.56-0.97), p=0.027).
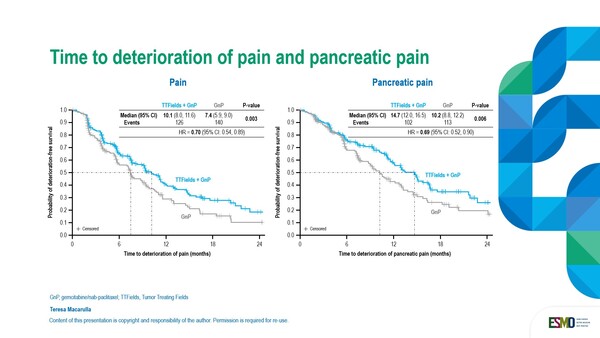
Additional analysis on quality of life data was presented at the ESMO congress in Barcelona. In the analysis of predefined secondary endpoints, QoL was assessed by using the EORTC QLQ C-30 questionnaire and PAN26 module, and health-related QoL deterioration-free survival (DFS) was also assessed. On the physical symptom scales, times to deterioration (worsening) of pain and pancreatic pain (p=0.003 and p=0.006, respectively) (Figure) were significantly longer with the TTFields combination compared to gemcitabine/nab-paclitaxel alone (median 10.1 versus 7.4 months for pain, and median 14.7 versus 10.2 months for pancreatic pain).
Pain medication use was analysed post-hoc. Findings showed that the addition of TTFields to the therapy regimen significantly prolonged time to meaningful increased pain medication (median 6.9 versus 4.9 months, p=0.049) and time to opioid use (7.1 versus 5.4 months, p=0.046, respectively). Time to deterioration in global health status was also significantly longer with TTFields (p=0.023).
Taken together, OS, QoL and pain management data favour the use of TTFields for the treatment of LAPAC. “However, a few open questions remain,” highlights Dr Angela Lamarca, Fundacion Jimenez Diaz, University Hospital in Madrid, Spain. “First, the study did not show an impact on PFS. This highlights potential challenges in response assessment of pancreatic lesions and makes us reflect on new potential techniques that could be utilized in this regard. The reality is that in the presence of a QoL improvement and an OS benefit, the absence of a PFS benefit feels less of a deal breaker if this device was to be approved for this indication. Secondly, concern remains regarding potential barriers to utilising the device, including the emotional and physical burden on the patient. In contrast, despite our perceptions, the available data on QoL does support its clinical benefit and its use in daily practice. Finally, the ways novel devices are usually incorporated into daily practice remain heterogenous across countries, and even among centres within the same country, and this may actually represent an additional challenge to face. The development of new devices in the field of oncology may require the implementation of novel logistic structures within clinical teams and a robust equity process.”