Study indicates that PARP inhibition could extend treatment options for patients with biliary tract cancers
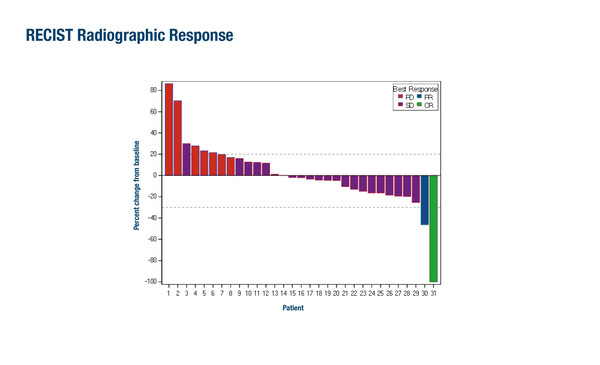
Phase II study results suggest encouraging efficacy for olaparib in patients with advanced biliary tract cancers who had received prior chemotherapy or refused chemotherapy and had aberrant germline or somatic homologous recombination repair (HRR) mutations (including PALB2, BRCA1/2 and ATM) (Abstract 261MO).
As reported at the ESMO Gastrointestinal Cancers Congress 2025 (Barcelona, 2–5 July), the study met its predefined threshold of success (≥19 of the first 30 evaluable patients were alive and free of progression at the first scan at ~8 weeks), with progression-free survival (PFS) achieved by 22 (73.3%) patients with olaparib. The observed objective response rate (ORR) was 6.5% and the disease control rate (DCR) was 74.2%. A complete response was observed in a patient with an ATM mutation and a partial response was observed in a patient with a germline BRCA1 mutation. Median overall survival was 11.2 months and median PFS was 16.7 weeks. Safety results showed that 25% of patients reported grade 3 or 4 treatment-related adverse events (TRAEs), particularly anaemia (grade 3; 12.5%).
Chemo-immunotherapy is the standard of care for patients with advanced biliary tract cancer, but for patients with refractory disease, there are few available treatment options. “Offering personalised therapy to those who are potentially sensitive to PARP inhibitors is a rational approach that has been successful in patients with pancreatic cancer and BRCA mutations. As we see here with olaparib, this premise also appears valid in biliary tract cancer,” notes Prof. Zev Wainberg from the David Geffen School of Medicine at UCLA, Los Angeles, CA, USA. “However, getting a better understanding of who may benefit is key: is response to olaparib dependent on germline or somatic mutations? is it dependent on BRCA mutations only or is response also seen in those with other mutations?” Wainberg also suggests that safety data could be important. “Newer agents with greater selectivity for PARP1 are in development, and these may have superior tolerability and efficacy profiles to olaparib,” he concludes.
Programme details
Ahn D, et al. A phase II study of olaparib in patients (pts) with advanced biliary tract cancer (aBTC) with aberrant homologous recombinant repair (HRR) mutations. ESMO Gastrointestinal Cancers Congress 2025, 261MO
Mini Oral Session, 05.07.2025, h. 08:30 – 10:00, Room Bilbao