According to results from several phase I trials, this precision oncology strategy also offers manageable safety
Claudins are transmembrane proteins that play a pivotal role in maintaining tight cell junctions. Several claudin isoforms are specifically expressed in solid tumours and it has been proposed that targeting these proteins with antibody–drug conjugates (ADCs) could be an effective and tolerable precision oncology strategy. At the ESMO Congress 2024 (Barcelona, 13–17 September), results were presented from three phase I trials that evaluated novel claudin-targeted ADCs in patients with advanced solid tumours.
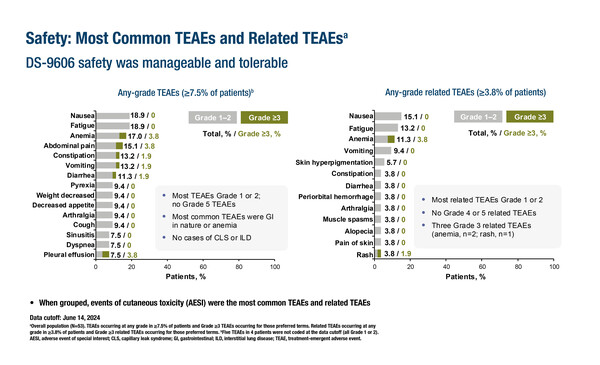
A claudin 6-directed ADC (DS-9606a) was evaluated in a phase I dose-escalation study in 53 patients with locally advanced or metastatic solid tumours (Abstract 610O). Treatment-related treatment-emergent adverse events (TEAEs) of any grade occurred in 28 (52.8%) patients, with the most common including anaemia (20.8%), abdominal pain (18.9%), nausea (18.9%) and fatigue (18.9%). Grade ≥3 treatment-related TEAEs were reported for 3 (5.7%) patients and there were two cases of grade ≥3 anaemia. Nine (17.0%) patients had TEAEs requiring treatment interruption, 3 (5.7%) patients required dose reduction, and 1 (1.9%) patient required treatment withdrawal. None led to death.
Commenting on these results, Dr Valentina Gambardella from the INCLIVA Biomedical Research Institute, University of Valencia, Spain, says, “This is a particularly interesting study as patients with several different tumour types were enrolled. It is encouraging to see that no dose-limiting toxicities (DLTs) were observed and that there was no neutropenia, which is a common and severe toxicity associated with many ADCs. While these are early results, the absence of severe toxicities and the potential specificity of the drug make it a promising candidate for further development.”
In a second study, the safety and efficacy of a novel ADC comprising a claudin 18.2-monoclonal antibody linked to a topoisomerase I inhibitor (SHR-A1904) was evaluated in 73 patients with gastric or gastroesophageal junction cancer (Abstract 609O). The majority of patients (98.6%) included in the study had metastatic disease and 31.5% were heavily pre-treated with three or more prior lines of therapy. Treatment-related TEAEs leading to dose reduction and treatment discontinuation were reported in 4 and 3 patients, respectively, during the dose-escalation phase. The maximum tolerated dose (MTD) was not reached during the study. Overall, grade ≥3 treatment-related AEs were reported for 39 (53.4%) patients, with the most common being anaemia and decreased white blood cell and neutrophil counts. For patients receiving the 6.0 mg/kg dose, the objective response rate (ORR) was 55.6% (5/9; 95% confidence interval [CI] 21.2–86.3) and the disease control rate (DCR) was 88.9% (8/9; 95% CI, 51.8–99.7). At a dose of 8 mg/kg, the ORR and DCR were 36.7% (11/30; 95% CI, 19.9–56.1) and 86.7% (26/30; 95% CI, 69.3–96.2), respectively. Gambardella notes, “In this heavily pre-treated population, DLTs were consistent with the toxicities associated with ADCs already used in clinical practice. The efficacy results are promising, and we look forward to seeing the results from the expansion phase of the study.”
Another claudin 18.2-targeting ADC with a topoisomerase I inhibitor payload (XNW27011) was investigated in a phase I/II study in 16 patients with locally advanced and/or solid tumours (Abstract 651P). During the dose-escalation phase, the most common AEs were nausea and vomiting, followed by anaemia and decreases in white blood cell and neutrophil counts. One patient experienced a DLT at 6.0 mg/kg. The ORR and DCR were 50% (7/14) and 86% (12/14), respectively, for all tumours. “This is a population of patients with predominantly gastrointestinal tumours that have a poor prognosis,” highlights Gambardella. “The safety profile in this study is encouraging and it will be very interesting to see how this ADC performs in a wider population.”
Gambardella concludes: “Targeting claudins represents a significant advance in the pursuit of precision oncology. The specificity of these targets, combined with the potency of ADCs, offers a promising approach to treating solid tumours that have become resistant to other forms of therapy. The early results are encouraging, but the full impact of these therapies will only become clear as more data emerge. These and other ongoing studies will be critical in determining whether these novel agents can fulfil their potential and become a standard of care for the treatment of solid tumours.”
Programme details
Yu J, et al. Phase I study of XNW27011, a novel claudin 18.2 ADC, in patients with locally advanced and/or metastatic solid tumors. ESMO Congress 2024, Abstract 651P
Poster Display – Developmental therapeutics, 14.09.2024, h. 12:00 – 13:00, Hall 6
Xu R-H, et al. CLDN18.2 targeted antibody-drug conjugate (ADC), SHR-A1904, in patients (pts) with gastric/gastroesophageal junction cancer (GC/GEJC): A phase I study. ESMO Congress 2024, Abstract 609O
Proffered Paper Session 2 – Developmental therapeutics, 15.09.2024, h. 14:45 – 16:15, Salamanca Auditorium – Hall 5
Patel MR, et al. Preliminary results from a phase I, first-in-human study of DS-9606A, a claudin 6 (CLDN6)-directed antibody–drug conjugate (ADC), in patients (pts) with tumor types known to express CLDN6. ESMO Congress 2024, Abstract 610O
Proffered Paper Session 2 – Developmental therapeutics, 15.09.2024, h. 14:45 – 16:15, Salamanca Auditorium – Hall 5