Study findings show that ctDNA is a stronger biomarker than carcinoembryonic antigen to select patients who may benefit from metastasis-directed therapy
Findings from a study presented at the ESMO Gastrointestinal Cancers 2025 suggested that adding circulating tumour (ct)DNA testing to the current standard of care in colorectal cancer (CRC) surveillance may help better identify patients who are candidates for curative-intent metastasis-directed therapy (MDT) (Abstract 2O).
Currently, standard of care surveillance includes serial history and physical examination, carcinoembryonic antigen (CEA), colonoscopy, and cross-sectional imaging for stages II-IV CRC. Whereas some studies have cast doubt on the value of such surveillance approach (JAMA Intern Med. 2025 Jun 1;185(6):710-719; J Clin Oncol. 2009 Aug 1;27(22):3671-6; Ann Oncol. 2015 Apr;26(4):644-656), ctDNA monitoring following resection demonstrated to provide valuable insight into recurrence and mortality risk stratification, offering a potential tool to guide adjuvant therapy decisions (Nat Med. 2024 Nov;30(11):3272-3283).
In the study, the rates of patients undergoing MDT stratified by ctDNA status were assessed in two large prospective observational studies in which serial ctDNA using a commercial tumour-informed assay (Signatera) was obtained during surveillance – GALAXY, which is an observational arm of the ongoing CIRCULATE-Japan study (Nat Med. 2023 Jan;29(1):127-134), and BESPOKE-CRC (J Clin Oncol 42, 9-9(2024).
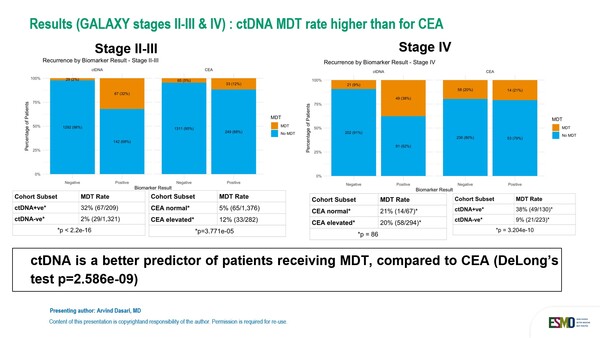
In the GALAXY study, which involved 1,883 patients with stage II-IV CRC, ctDNA showed to be a better predictor of patients receiving curative-intent metastasis-directed therapy compared to carcinoembryonic antigen. When stratified by longitudinal ctDNA status, 32.0% of ctDNA-positive patients in stage II-III received MDT versus only 2% (29/1312) of ctDNA-negative, while differences were smaller when patients were stratified by CEA levels, with 12% of patients with elevated levels of CEA receiving MDT versus 5% of those with CEA levels within normal limits. In stage IV CRC patients, the rates were 38% for ctDNA-positive patients versus 9% for ctDNA-negative patients, and 20% for CEA-elevated patients versus 21% for CEA-normal patients. Risk of relapse was minimal to lower in patients with persistently negative ctDNA results in the stage II-III and stage IV cohorts respectively.
Similar results were observed in the BESPOKE-CRC study involving 1,221 patients with stage II-III CRC, with 22% of ctDNA-positive patients receiving MDT compared to only 1% of those who were ctDNA-negative.
Findings presented support the addition of ctDNA testing to personalise current surveillance strategy in CRC and better identify patients who may benefit from MDT.