The first results from two phase III studies of Ra-223 and 177Lu-PNT2002 may help to decipher their role in the treatment of patients with mCRPC
Despite being used for decades in the palliative treatment of prostate cancer, recent times have seen a revival in interest in radiopharmaceuticals after positive findings were reported in metastatic castration-resistant prostate cancer (mCRPC).
At the ESMO Congress 2024 (Barcelona, 13–17 September) Late-Breaking results provide evidence of efficacy benefit in this unmet need population using novel therapeutic approaches involving the bone-targeting radioisotope radium (Ra)-223 and a PSMA-targeting small molecular ligand 177Lu-PNT2002, in addition to, or versus, an androgen receptor pathway inhibitor (ARPI), one of the key treatments for this cancer.
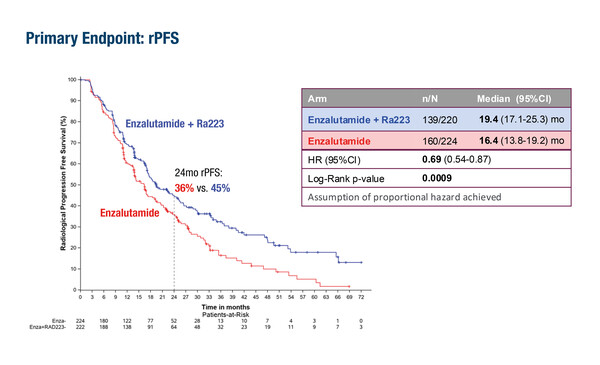
In a Presidential Symposium, results were presented from the randomised, multicentre, open-label PEACE-3 trial of the ARPI enzalutamide plus the bone-targeting radioisotope radium (Ra)-223 compared with enzalutamide alone in 446 men with asymptomatic or mildly symptomatic mCRPC and bone metastases (LBA1). At a median follow-up of 42.2 months, the study was positive for the primary endpoint of radiological progression-free survival (rPFS), with median rPFS of 19.4 months (95% confidence interval [CI] 17.1–25.3) in the enzalutamide plus Ra-223 arm and 16.4 months (95% CI 13.8–19.2; hazard ratio [HR] 0.69; p=0.0009) in the enzalutamide-only arm. Overall survival (OS) was also considered superior in the enzalutamide plus Ra-223 arm compared with the enzalutamide-only arm at the pre-planned interim analysis (HR 0.69; p=0.0031).
Treatment-emergent adverse events (TEAEs) of any grade were reported at a similar frequency in the enzalutamide plus Ra-223 and the enzalutamide-only arms (100% and 96.4%, respectively), while grade ≥3 TEAEs were more common in the enzalutamide plus Ra-223 arm (65.6% versus 55.8%, respectively). Commenting on the study results, Prof. Karim Fizazi from Institut Gustave Roussy and University of Paris Saclay, Villejuif, France highlights, “Importantly, OS seems to be improved with enzalutamide plus Ra-223, with an approximately 30% reduction in risk of progression or death, and the analysis also showed acceptable safety for the combination.” While positive findings from the final analysis of the PEACE-3 study may be expected based on the presented data, Fizazi cautions that whether the PEACE-3 data are practically useful will mostly depend on how ARPIs are used: in the trial, enzalutamide was used for mCRPC. “However, there is now robust phase III evidence that we should actually use ARPIs in earlier-stage disease, e.g. when the disease remains castration-sensitive. For clinicians who normally reserve ARPIs for mCRPC, the PEACE-3 results provide evidence of a new and more efficacious treatment option for earlier-stage disease,” he adds.
In a second study presented at the Congress, initial results of a prostate-specific membrane antigen (PSMA)-based therapy in the open-label, multicentre SPLASH study were also reported (LBA65). The study enrolled 412 men with PSMA-positive mCRPC who had cancer progression on one ARPI and had not received chemotherapy for castration-resistant disease, and were randomised to receive either a beta-emitting radioisotope chelated to 177Lu-PNT2002 (n=276) or an alternative ARPI (n=136). The primary endpoint of rPFS was improved with 177Lu-PNT2002 compared with the alternative ARPI (9.5 months versus 6.0 months; HR 0.71; p=0.0088). Reflecting on these results, Fizazi says, “While the early results appear positive, the final trial results are eagerly anticipated, as the initial magnitude of benefit is perhaps not as great as hoped. Although making comparisons across studies is not always advised, the results of 177Lu-PNT2002 in the SPLASH trial will need to be considered in light of the findings for 177Lu-PSMA-617, particularly in the VISION study (N Engl J Med. 2021;385:1091–1103), which led to 177Lu-PSMA-617 becoming a standard of care in men with mCRPC who have undergone chemotherapy, and in the PSMAfore study, presented at the ESMO Congress 2023, which had a very similar patient population to that of the SPLASH study. Once we have the mature data from the SPLASH and PSMAfore studies, determining the clinical relevance of 177Lu-PNT2002 and 177Lu-PSMA-617 will become easier.”
Reflecting on the progress made in the metastatic prostate cancer setting over the past two decades, Fizazi acknowledges that many challenges remain. “There has been a fundamental improvement in outcomes for patients; however, we are not yet able to offer our patients a cure. We need more evidence to determine whether radiopharmaceuticals will play a role to this effect.” In terms of PSMA-targeted therapies, research is underway for 177Lu-PSMA-based treatments including the final results of the PSMAfore and SPLASH trials, as well as studies in earlier settings, including PSMAddition in metastatic hormone-sensitive disease. Fizazi thinks that more evidence is needed to understand the place of Ra-223 in the treatment pathway relative to other treatments in the armamentarium. “The PEACE-3 trial affirms its value and other ongoing trials, including a phase IV trial of Ra-223 (RADIANT), will help clarify its position in the care pathway. Additionally, ongoing research into different radioisotopes and different targets also offer new hope for future treatments,” he concludes.
Programme details
Gillessen S, et al. A randomized multicenter open label phase III trial comparing enzalutamide vs a combination of Radium-223 (Ra223) and enzalutamide in asymptomatic or mildly symptomatic patients with bone metastatic castration-resistant prostate cancer (mCRPC): First results of EORTC-GUCG 1333/PEACE-3. ESMO Congress 2024, LBA1
Presidential Symposium I – Practice-changing trials, 14.09.2024, h. 16:30 – 18:15, Barcelona Auditorium – Hall 2
Sartor O, et al. Efficacy of 177Lu-PNT2002 in PSMA-positive mCRPC following progression on an androgen-receptor pathway inhibitor (ARPI) (SPLASH). ESMO Congress 2024, LBA65
Proffered Paper Session – GU tumours, prostate, 15.09.2024, h. 14:45 – 16:15, Valencia Auditorium – Hall 5