In the IMvigor011 trial, ctDNA positivity was reported to be strongly predictive of adjuvant immunotherapy benefit in muscle-invasive bladder cancer
“We now have the first level 1 evidence from a prospective, randomised study in muscle-invasive bladder cancer (MIBC) that a patient should be treated when molecular residual disease (MRD) is detected, and spared treatment – and its associated toxicities – when MRD is persistently absent,” says Dr Alexander Wyatt from the University of British Columbia, Vancouver, Canada, commenting on today’s Presidential Symposium results from the IMvigor011 study (LBA8). The trial demonstrated significant survival benefits with adjuvant atezolizumab versus placebo in 250 patients who tested positive for circulating tumour DNA (ctDNA) after radical cystectomy for MIBC.
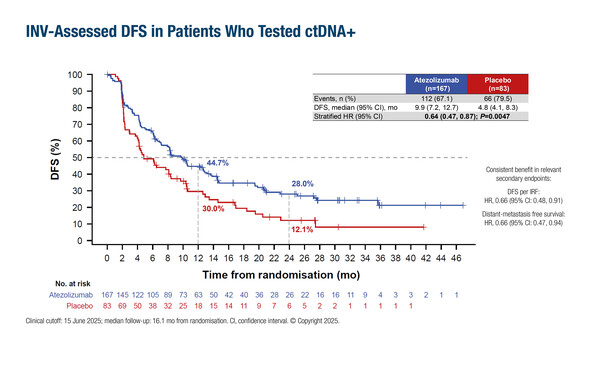
As presented at the ESMO Congress 2025 (Berlin, 17–21 October), at a median follow-up of 16.1 months, patients in the atezolizumab arm had a median investigator-assessed disease-free survival (DFS; the primary endpoint) of 9.9 months versus 4.8 months with placebo (hazard ratio [HR] 0.64; 95% confidence interval [CI] 0.47–0.87; p=0.0047), and a median overall survival (OS) of 32.8 months versus 21.1 months with placebo (HR 0.59; 95% CI 0.39–0.90; p=0.0131).
The 12-month DFS rate was 44.7% with atezolizumab versus 30.0% with placebo, and the corresponding 12-month OS rates were 85.1% versus 70.0%. For comparison, among 357 patients who persistently tested ctDNA-negative after serial MRD testing, the 12-month DFS rate was 95.4% and the 12-month OS rate was 100%. At 24 months, DFS and OS rates in ctDNA-negative patients were 88.4% and 97.1%, respectively. Treatment-related grade 3–4 adverse events in ctDNA-positive patients occurred in 7.3% of those in the atezolizumab arm versus 3.6% in the placebo arm, while 3 treatment-related deaths were reported with atezolizumab and none with placebo.
The data support a growing body of evidence that ctDNA-based detection of MRD is strongly prognostic after cystectomy (J Clin Oncol. 2019;37:1547–1557; J Clin Oncol. 2025;43(Suppl):4503). “There is a hypothesis that if you can detect ctDNA in a patient’s blood despite no other radiological evidence of cancer, you should intervene,” notes Wyatt. This is largely based on retrospective data from an earlier IMvigor010 study suggesting that patients who tested ctDNA-positive after cystectomy derived benefit from adjuvant atezolizumab, while patients who tested ctDNA-negative did not (Nature 2021;595:432–437; Eur Urol. 2024;85:114–122).
While statistically significant, Wyatt acknowledges that the clinical impact of the data from IMvigor011 should be interpreted in the context of the treatment and disease studied: “Adjuvant atezolizumab is actually not widely used in the treatment of bladder cancer. The landscape is changing rapidly, both in the adjuvant and neoadjuvant settings, and we cannot immediately extrapolate the findings to other immunotherapies or to antibody–drug conjugates or to other types of solid cancer. However, the data open the door to further trials, including across the spectrum of peri-operative therapy for bladder cancer,” he says. Studies such as NIAGARA (J Clin Oncol. 2025;43(Suppl):4503), TOMBOLA (NCT04138628) and NEO-BLAST (NCT06537154) are generating evidence in this space, while others, such as the MODERN trial (NCT05987241), are investigating whether ctDNA may be used to guide adjuvant therapy escalation and de-escalation post-surgery.
Wyatt notes that the IMvigor011 findings should also be considered in relation to the main test (SignateraTM) used in the trial to detect ctDNA. “This is an earlier generation assay that has been available for some time, so there is a good level of familiarity with it. But there has been criticism that the test has lower sensitivity than some of the newer assays that leverage the whole genome rather than an exome,” he explains. There may be uncertainty as to whether this test should be adopted to study different indications moving forward. However, Wyatt says that the lower sensitivity of the assay was mitigated to some extent in IMvigor011 through the use of serial MRD monitoring: “This provides additional opportunities to identify ctDNA-positive patients, so it is a clever design and I think we have to be careful that perfection does not become the enemy of progress. We do not strive for 100% accuracy with other diagnostics such as imaging,” he observes.
Programme details:
Powles TB, et al. IMvigor011: a Phase 3 trial of circulating tumour (ct)DNA-guided adjuvant atezolizumab vs placebo in muscle-invasive bladder cancer. ESMO Congress 2025 - LBA8