However, lack of OS benefit limits the clinical impact of the findings from the PSMAfore trial
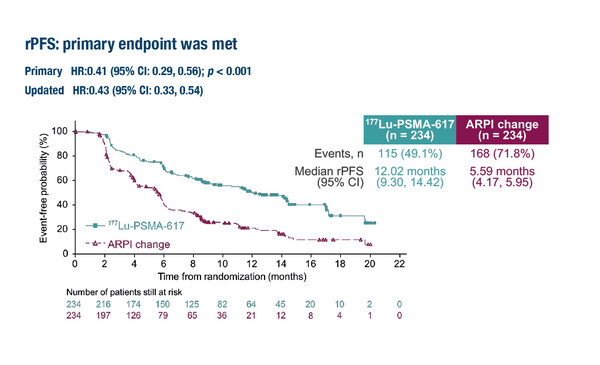
According to results presented in a Presidential Session at the ESMO Congress 2023 (Madrid, 20–24 October), the PSMAfore trial met its primary endpoint, demonstrating an improvement in radiographic progression-free survival (rPFS) with the prostate-specific membrane antigen (PSMA)-targeted radioligand 177Lu-PSMA-617 compared with androgen receptor pathway inhibitor (ARPI) switch in metastatic castration-resistant prostate cancer (mCRPC) progressing on prior ARPI therapy (LBA13).
The trial involved 468 patients with taxane-naïve mCRPC and at least one PSMA-positive lesion. At a median follow-up of 7.3 months, the primary analysis demonstrated a 59% reduction in the risk of radiographic progression with 177Lu-PSMA-617 versus ARPI change (abiraterone/enzalutamide) (hazard ratio [HR] 0.41; 95% confidence interval [CI] 0.29–0.56; p<0.0001). This was confirmed by a second interim analysis at a median follow-up of 15.9 months, which reported median rPFS for 177Lu-PSMA-617 and ARPI switch of 12.02 months and 5.59 months, respectively (HR 0.43; 95% CI 0.33–0.54; p<0.0001).
Patients randomised to sequential ARPI could cross over to 177Lu-PSMA-617 following centrally reviewed radiographic disease progression. At the second interim analysis, 84.2% of the 146 patients with radiographic disease progression on ARPI had crossed over to 177Lu-PSMA-617.
“The results from the PSMAfore trial are interesting, but their impact on clinical practice will be limited without confirmation of an overall survival (OS) benefit,” suggests Prof. Nicholas James from the Institute of Cancer Research and Royal Marsden Hospital, London, UK, commenting on the results. In the trial, a trend for an OS benefit of 177Lu-PSMA-617 over ARPI switch was observed when data were analysed according to the pre-specified crossover adjusted analysis, but not when the analysis was unadjusted. Objective response rates in patients with soft tissue only disease were 50.7% for 177Lu-PSMA-617 and 14.9% for ARPI switch, with corresponding median durations of responses of 13.6 months and 10.1 months, respectively. 177Lu-PSMA-617 was associated with an increase in the median time to worsening Functional Assessment of Cancer Therapy-Prostate total score (7.46 months versus 4.27 months; HR 0.59). The incidence of grade ≥3 adverse events (AEs), serious AEs and AEs leading to treatment discontinuation were 34%, 20% and 5.7%, respectively, with 177Lu-PSMA-617, and 43%, 28% and 5.2%, respectively, with ARPI switch.
“We also need to consider cost and the possibility that treatment in the comparator arm was suboptimal. Switching from one ARPI to an alternative is known to be a strategy with limited efficacy compared to switching to chemotherapy (N Engl J Med. 2019;381:2506–2518), although considerations around a patient’s fitness for chemotherapy needs to be taken into account. If a patient is fit for chemotherapy, this trial is essentially comparing radio-isotope therapy followed by chemotherapy with the reverse sequence, so it is hard to see much gain from earlier scheduling. If a patient is not fit for chemotherapy, the results may be of greater interest given the reported side-effect profile in this study as well as pre-existing data,” continues James. Earlier this year, 177Lu-PSMA-617 was included in an update of ESMO guidelines for the treatment of metastatic relapsed prostate cancer pre-treated with a novel ARPI and taxanes, based on the results of the phase III VISION trial (Ann Oncol. 2023;34:557–563). “177Lu-PSMA-617 is an attractive agent with a good toxicity profile in this setting. Its use upfront early in the course of the prostate cancer, when PSMA-negative lesions are rare, may be more encouraging and it will be interesting to see the results from the ongoing phase III PSMAddition trial (NCT04720157) and the imminent STAMPEDE2 trial (Clin Oncol (R Coll Radiol). 2023;35:e628–e635) in this setting,” he concludes.
Abstract discussed:
Sartor O, et al. Phase 3 trial of [177Lu]Lu-PSMA-617 in taxane-naive patients with metastatic castration-resistant prostate cancer (PSMAfore). ESMO Congress 2023, LBA13
Presidential Symposium 3, 23.10.2023, h. 16:30 – 18:15, Madrid Auditorium – Hall 6