The combination of lenvatinib plus everolimus improved progression-free survival in the LenCabo trial, but toxicity must be carefully assessed
In a head-to-head randomised comparison of contemporary second-line treatments after progression on PD-1 immune checkpoint inhibition (ICI), lenvatinib plus everolimus significantly prolonged progression-free survival (PFS) over cabozantinib in patients with metastatic clear cell renal cell carcinoma (ccRCC) (LBA94).
Second-line treatment for metastatic ccRCC depends on the first-line therapy administered. The ESMO Living Guideline in this tumour type sets sequencing vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor (TKI) therapy after PD-1-targeted first-line therapy as the standard of care. VEGFR-targeted agents that have not been previously used should be considered in the second-line setting, with cabozantinib as the preferred agent, and other agents representing alternative options. “Over the last 20 years, we have seen vastly improved outcomes from advanced RCC with the introduction of therapies that target the VEGF axis and the PD1 checkpoint, and the ability to use those both in sequence and in combination,” said Dr Lisa M. Pickering of the Royal Marsden Hospital, London, UK, who discussed the results from the multicenter phase II LenCabo trial at the ESMO Congress 2025.
Although cabozantinib and lenvatinib share many kinase targets, lenvatinib also blocks FGFR and is paired with the mTOR inhibitor everolimus, potentially overcoming additional resistance pathways. “So, the LenCabo asks real-life, pragmatic questions: how to best treat patients who were previously treated with an ICI, and is there a difference between lenvatinib plus everolimus at standard dosing, over cabozantinib?”
As presented at the ESMO Congress, the study randomised 90 patients with metastatic ccRCC to either receive lenvatinib (18 mg/d) plus everolimus (5 mg/d) versus cabozantinib (60 mg/d) after 1-2 prior lines of treatment, including a PD-1 ICI. Median PFS was 15.7 months with levatinib plus everolimus and 10.2 months with cabozatinib [hazard ratio (HR) 0.51; 95% CI 0.29–0.89, p=0.02]. The objective response rate (ORR) was 52.6% with the dual targeted treatment compared to 38.6% with cabozantinib. Overall survival (OS) data was immature.
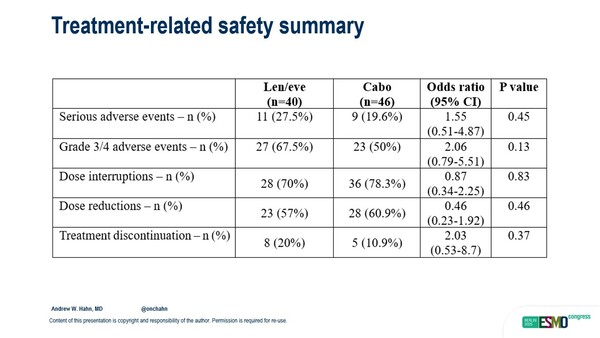
“The trial populations in this trial were pretty well balanced, with just some minor differences suggesting that the results did not happen because the lenvatinib-everolimus group had less aggressive or more treatable cancers,” Pickering noted. “It is important to recall that there was higher grade 3-4 toxicity and treatment discontinuation with the combination, and that is really important for a group of patients with advanced cancer being treated in the second- and third-line setting.”
Regarding the potentially practice-changing impact of these results, Pickering concluded: “I feel I already know that lenvatinib plus everolimus is a highly active regimen, but it has been less used while cabozatinib is very widely used in this setting. So, are these results positive enough to change practice? I think that depends on you and your patient, but I would say that we can and should consider using lenvatinib plus everolimus, particularly in patients for whom the priority is response, and for whom the higher toxicity rate is acceptable.”