Presidential Symposium presentation provides insights into the role of 177Lu-PSMA-617 in the treatment of hormone-sensitive prostate cancer, with results also presented in castration-resistant disease
There is a strong rationale for adding 177Lu-PSMA-617 to androgen deprivation therapy (ADT) plus an androgen receptor pathway inhibitor (ARPI) because of the high expression of prostate-specific membrane antigen (PSMA) in prostate cancer cells and the good activity already seen in the metastatic castration-resistant prostate cancer (mCRPC) setting (N Engl J Med. 2021;385:1091–1103).
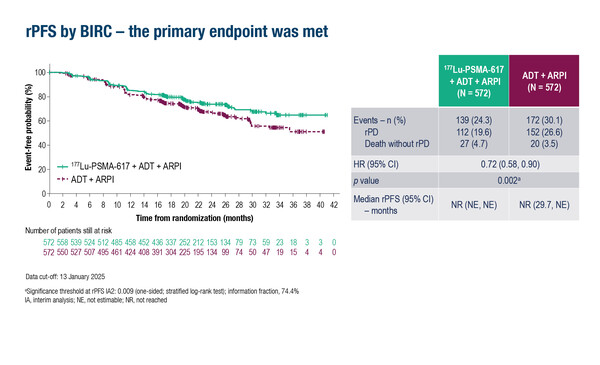
Evidence was further supported by the phase III PSMAddition trial, which demonstrated that adding 177Lu-PSMA-617 to ADT plus ARPI significantly improved radiographic progression-free survival (rPFS) – the primary endpoint – compared with ADT plus ARPI in 1,144 patients with prostate-specific membrane antigen (PSMA)-positive metastatic hormone-sensitive prostate cancer (mHSPC) at a median 23.6 months of follow-up (LBA6, key results in the box below). According to data discussed at today’s Presidential Symposium at the ESMO Congress 2025 (Berlin, 17–21 October), the rPFS benefit with the 177Lu-PSMA-617-based regimen was consistent across subgroups, including high and low tumour volume and de novo and recurrent metastatic disease, and regardless of previous prostatectomy or definitive radiotherapy.
Despite the study being positive, Prof. Arun Azad from the University of Melbourne and Peter MacCallum Cancer Centre, Melbourne, Australia, cautions against assessing its impact on current clinical practice until overall survival (OS) data are mature (interim analysis: hazard ratio [HR] 0.84; 95% confidence interval [CI] 0.63–1.13; p=0.125). “At present, ~47% of the OS events have occurred, but the data do not indicate significance at this stage,” he adds. “While a positive study that meets its primary endpoint will often lead to regulatory approval, what we really want is to change outcomes for patients in clinical practice and mature OS analysis will help assess the magnitude of benefit of the triple combination.”
In this trial, treatment-related adverse events were more frequent with the 177Lu-PSMA-617 combination than with ADT plus ARPI (89.4% versus 69.7%), although there was no obvious difference in quality of life detriment between study arms, and events associated with 177Lu-PSMA-617 were as expected (e.g. dry mouth, fatigue, nausea and cytopenia). “However, there are long-term toxicities associated with 177Lu that typically take longer than 2 years to emerge and include secondary myeloid neoplasms, such as myelodysplastic syndrome, and renal impairment. These may be rare, but they are important to track over the next 5–10 years,” cautions Azad.
The PSMAddition trial allowed patients initially randomised to the control arm to switch to 177Lu-PSMA-617 upon radiographic disease progression, enabling analysis of outcomes by treatment sequence in the mHSPC setting. A second study presented at the ESMO Congress, the randomised phase II PR21 trial, showed that in chemotherapy-naïve patients with mCRPC progressing after ARPI therapy, switching to 177Lu-PSMA-617 did not significantly improve rPFS compared with switching to docetaxel (8.6 months versus 10.7 months, respectively; p=0.51), whereas OS was significantly longer after switching to docetaxel rather than 177Lu-PSMA-617 (18.2 months versus 14.3 months, respectively; p=0.02) (LBA89, key results in the box below). Commenting on these findings, Azad says, “This study reinforces two things. Firstly, that docetaxel remains a very good therapy for prostate cancer, although often overlooked, perhaps because of its associated toxicity. Secondly, that using 177Lu-PSMA-617 earlier may not lead to improved survival in chemotherapy-fit patients with mCRPC.”
The place of 177Lu-PSMA-617 in the treatment of prostate cancer is still uncertain. Azad says that more work needs to be done to determine which patients may benefit the most. “Further insights may come from imaging analyses; for instance, mean standardised uptake value (SUVmean) was identified as a predictive biomarker for the efficacy of 177Lu-PSMA-617 in the VISION and TheraP trials (Radiology. 2024;312:e233460; Lancet Oncol. 2022;23:1389–1397). Also, there is currently a broad definition of PSMA positivity, but a concerted effort to identify a specific level of target expression that makes those tumours more likely to respond to 177Lu-PSMA-617 therapy could help personalise therapy and optimise treatment decisions in the future.”
At a glance:
Tagawa ST, et al. Phase 3 trial of [177Lu]Lu-PSMA-617 combined with ADT + ARPI in patients with PSMA-positive metastatic hormone-sensitive prostate cancer (PSMAddition). ESMO Congress 2025 - LBA6
- N=1,144 (177Lu-PSMA-617 + ADT + ARPI n=572; ADT + ARPI n=572)
- 177Lu-PSMA-617 + ADT + ARPI vs ADT + ARPI
- Median rPFS: NR vs NR; HR 0.72; 95% CI 0.58–0.90; p=0.002
- Interim OS: NR vs NR; HR 0.84; 95% CI 0.63–1.13; p=0.125
- TRAEs: 89.4% vs 69.7%; grade ≥3 TRAEs: 22.7% vs 12.2%
Chi KN, et al. A randomized phase 2 study of 177Lu-PSMA-617 vs docetaxel in patients with metastatic castration-resistant prostate cancer (mCRPC) and PSMA-positive disease: Canadian Cancer Trials Group (CCTG) study PR.21. ESMO Congress 2025 - LBA89
- N=199 (177Lu-PSMA-617 n=100; docetaxel n=99)
- 177Lu-PSMA-617 vs docetaxel:
- rPFS: 8.6 mo vs 10.7 mo; HR 1.01; 90% CI 0.77–1.31; 1-sided p=0.51
- OS: 14.3 mo vs 18.2 mo; HR 1.64; 90% CI 1.14–2.35; 2-sided p=0.02
- CR/PR (pts with measurable disease): 16% vs 8%; p=0.23
- Grade 3-4 TRAEs: 13% vs 34%