In the CAPItello281 trial, survival results with capivasertib plus abiraterone are promising, but questions remain on the accurate determination of PTEN loss in patients
The pan-AKT kinase inhibitor capivasertib is approved both in Europe and in the USA in combination with fulvestrant for the treatment of hormone receptor-positive metastatic breast cancer with PTEN, AKT1 or PIK3CA gene mutations. Currently, no AKT inhibitor has yet been approved for the treatment of prostate cancer.
Approximately 30% of patients with hormone-sensitive prostate cancer (HSPC) have PTEN-deficient tumours, resulting in dysregulation of PI3K/AKT and androgen receptor (AR) signalling. “This patient population experiences reduced clinical benefit from AR pathway inhibition than those without alterations, resulting in a shorter time to disease progression. There is clearly an unmet need for more effective targeted treatment, which are eagerly awaited,” explains Dr Elena Castro from the Hospital Universitario 12 de Octubre, Madrid, Spain, considering the emerging data from the combination of capivasertib with abiraterone, which has the potential to change current clinical practice.
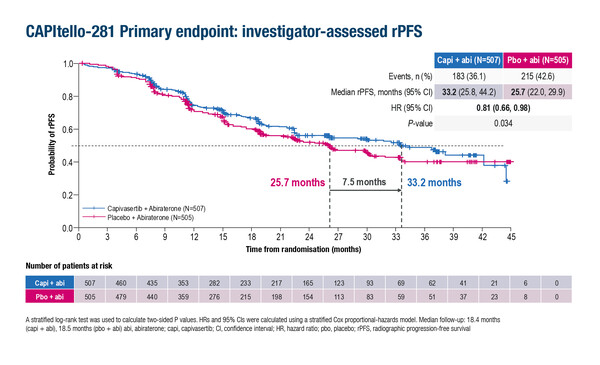
As reported from the phase III CAPItello-281 study at the ESMO Congress 2025 (Berlin, 17–21 October), a statistically significant improvement in radiographic progression-free survival (rPFS) was observed with the addition of capivasertib to abiraterone compared with placebo plus abiraterone in 1,012 patients with de novo PTEN-deficient metastatic hormone-sensitive prostate cancer (mHSPC) (33.2 months versus 25.7 months, respectively; hazard ratio [HR] 0.81; 95% confidence interval [CI] 0.66–0.98; p=0.034) (Abstract 2383O).
Castro comments that the degree of rPFS benefit with the capivasertib combination appeared to depend on the threshold used to define PTEN loss. “This was defined in the study as ≥90% of viable malignant cells with no specific cytoplasmic staining by immunohistochemistry (IHC), but increasing benefit was observed in subgroups selected using progressively more stringent PTEN cut-offs,” she explains. HRs ranged from 0.75 to 0.68 for ≥95% and 100% of viable malignant cells, respectively. “Despite having a very clear target that is present in a substantial proportion of patients with prostate cancer, accurately determining PTEN loss can be challenging; we are not sure of the right cut-off if using IHC, and previous studies (J Clin Oncol. 2022;40(Suppl 16):5056) have suggested that next-generation sequencing may be a more accurate technique.”
Regarding the overall survival benefit of capivasertib, interim results suggest a numerical advantage for the capivasertib combination versus the placebo arm (HR 0.90; 95% CI 0.71–1.15; p=0.401), but further data are needed.
Although findings from the CAPItello-281 study are encouraging, Castro is cautious about their potential impact on current clinical practice. “Before the combination therapy enters standard care, it will be important to establish a clear survival advantage and also agreement on the most accurate method to establish PTEN loss to determine those patients who will really benefit from this treatment,” she concludes.
Programme details:
Fizazi K, et al. A Phase 3 study of capivasertib (capi) + abiraterone (abi) vs placebo (pbo) + abi in patients (pts) with PTEN deficient de novo metastatic hormone-sensitive prostate cancer (mHSPC): CAPItello-281. ESMO Congress 2025 - Abstract 2383O