The combination, incorporating the novel antibody–drug conjugate, may transform first-line treatment in the setting, but crucial issues need to be addressed
Combining an antibody–drug conjugate (ADC) with immunotherapy marks a major advance in the treatment of diverse tumour types, including urothelial carcinoma. Survival rates in patients with HER2-expressing locally advanced or metastatic urothelial carcinoma nearly doubled by combining the HER2-targeting ADC disitamab vedotin with the PD-1 inhibitor toripalimab as first-line treatment for patients compared with chemotherapy, according to a Presidential Symposium presentation at the ESMO Congress 2025 (Berlin, 17–21 October).
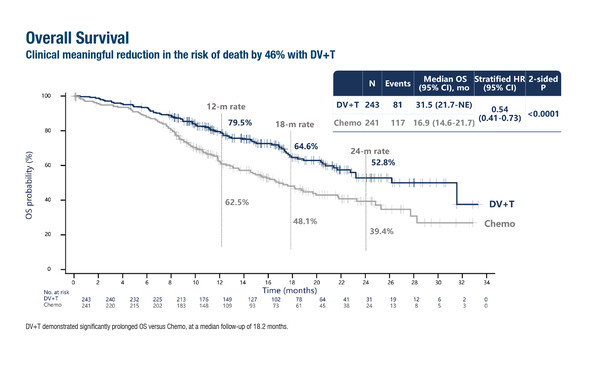
In the study, median overall survival – a co-primary endpoint – was 31.5 months in patients treated with disitamab vedotin plus toripalimab (n=243) compared with 16.9 months for those receiving chemotherapy (n=241; hazard ratio [HR] 0.54; 95% confidence interval [CI] 0.41–0.73; p<0.0001) at a median follow-up of 18.2 months in the phase III RC48-C016 trial (LBA7). Similarly, significant improvements were also reported for the other primary endpoint, median progression-free survival (13.1 months versus 6.5 months, respectively; HR 0.36; 95% CI 0.28–0.46; p<0.0001). Objective response rates were 76.1% with disitamab vedotin plus toripalimab and 50.2% with chemotherapy.
These positive findings are in line with data previously reported in the phase III EV-302/KEYNOTE-A39 trial of enfortumab vedotin plus pembrolizumab (N Engl J Med. 2024;390:875–888), and “position the regimen with disitamab vedotin as a highly promising option with the potential to change clinical practice,” says Prof. Andrea Necchi from Vita-Salute San Raffaele University, Milan, Italy.
Fewer patients in the disitamab vedotin plus toripalimab arm experienced grade ≥3 treatment-related adverse events (TRAE; 55.1% versus 86.9% in the chemotherapy arm). Of note, the spectrum of severe TRAE included a proportion of 4.1% peripheral neuropathy in the disitamab vedotin plus toripalimab arm, which is generally consistent with the EV-302/KEYNOTE-A39 trial. In general, there were more adverse events involving hepatic function test alterations and haematological toxicity in particular with disitamab vedotin plus toripalimab than with enfortumab vedotin plus pembrolizumab.
As ADC–immunotherapy combinations move toward approval, the development of robust companion diagnostics will be critical, as well as the turnaround time for biomarker test results. Especially for patients presenting with symptomatic disease or rapidly progressive tumour, for which there could be effective therapeutic options immediately available as therapeutic standards. “There are several limitations to consider,” continues Necchi. “Firstly, the RC48-C016 study was conducted exclusively in a Chinese population, implying that further similar studies involving a wider range of race and ethnicities by covering a broader spectrum of geographical areas are needed. Secondly, in a rapidly evolving field with newly approved standard-of-care treatments, platinum-based chemotherapy already represents an outdated arm in countries where the combination of enfortumab and pembrolizumab is available and reimbursed.” He notes that patients have become increasingly nervous to be screened within studies randomising them towards the chemotherapy alone option. Additionally, variability in HER2 biomarker evaluation remains an important consideration. Treatment sequencing is another important issue as the therapeutic landscape of urothelial cancer becomes more complex. “We do not yet know how disitamab vedotin with toripalimab will perform in patients previously exposed to ADCs or checkpoint inhibitors at earlier disease stages. With adjuvant immunotherapy already a therapeutic option in this setting, achieving clinical benefits in pre-treated patients may be more challenging than in treatment-naïve patients,” adds Necchi. Notably, earlier this year, the same Chinese authors presented preliminary results of a peri-operative disitamab vedotin–toripalimab combination in patients with muscle-invasive bladder cancer (J Clin Oncol. 2025;43(Suppl 5);665).
Ongoing studies may help address these knowledge gaps. Globally, multiple trials are evaluating ADC–immunotherapy combinations across different disease stages. In the first-line setting, a phase III trial of disitamab vedotin plus pembrolizumab versus platinum-based chemotherapy with maintenance immunotherapy is recruiting patients in multiple regions (J Clin Oncol. 2024;42(Suppl 4):TPS717) and this should pave the way for broader approval of disitamab vedotin beyond China. “Finally, biomarker-based patient selection remains a key focus,” concludes Necchi. “Both HER2 and NECTIN-4 are enriched in luminal subtype tumours, suggesting that the patient populations benefiting from disitamab vedotin and enfortumab vedotin may overlap. Developing novel therapies that target new biomarkers, as well as sequencing ADCs with non cross-resistant payloads, will be essential to further differentiate treatment options in the future and broaden the range of patients who can benefit from ADC–immunotherapy combinations.”
Programme details:
Sheng X, et al. Disitamab vedotin (DV) plus toripalimab (T) versus chemotherapy (C) in first-line (1L) locally advanced or metastatic urothelial carcinoma (la/mUC) with HER2-expression. ESMO Congress 2025 - LBA7