Two studies hint at a lack of effect of palbociclib on ovarian function and demonstrate similar reduced recurrence with abemaciclib regardless of menopausal status
Two presentations at ESMO Breast Cancer 2022 provide insights into important questions that are critical for the counselling and treatment of young women with early-stage breast cancer – do CDK4/6 inhibitors affect fertility and are they beneficial in premenopausal women?
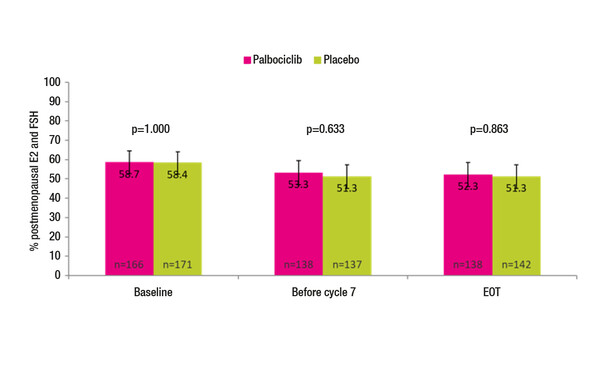
As presented at a Mini Oral Session, an exploratory analysis from the phase III PENELOPE-B trial appeared to show no impact on hormone levels when palbociclib was added to endocrine therapy in 576 evaluable young patients with hormone receptor (HR)-positive, HER2-negative high-risk early breast cancer after neoadjuvant chemotherapy (Abstract 60MO). Levels of oestradiol, follicle-stimulating hormone and anti-Müllerian hormone (a measure of ovarian reserves) were not significantly different after the end of treatment with palbociclib plus endocrine therapy compared with baseline. In total, 41.6% of patients in the placebo arm versus 41.3% in the palbociclib arm had premenopausal hormone levels at baseline, and after the end of treatment, the respective proportions with premenopausal hormone levels were 48.7% versus 47.7% (p=0.863) (Figure).
“Although the effects of different types of chemotherapy regimens on ovarian function are well known, there are very few data on the reproductive safety of new targeted agents including CDK4/6 inhibitors,” says Associate Prof. Matteo Lambertini from the University of Genova, Italy. “This is a crucial question, given that women who receive adjuvant CDK4/6 inhibitors are already at high risk of ovarian toxicity due to prior chemotherapy.” Discussing the specific results from the PENELOPE-B trial, he comments, “This exploratory analysis is important as it is the first to report on this topic; however, based on the data on hormone levels presented, it is premature to conclude that CDK4/6 inhibitors do not affect ovarian function: more long-term, comprehensive data are needed.” As the combination of palbociclib with endocrine therapy did not improve invasive disease-free survival (iDFS) versus endocrine therapy alone in the PENELOPE-B trial (J Clin Oncol. 2021;39:1518–1530), Lambertini questioned the applicability of these findings to clinical practice.
A poster presentation at ESMO Breast Cancer 2022 sheds some light on the efficacy of another CDK4/6 inhibitor, abemaciclib, in combination with endocrine therapy in young women. A subgroup analysis of the monarchE trial found treatment benefits with the addition of abemaciclib to endocrine therapy in women with HR-positive, HER2-negative early breast cancer at high risk of relapse were similar in the 43.5% of women who were premenopausal and the 56.4% who were postmenopausal (Abstract 63P). The hazard ratio for iDFS for abemaciclib plus endocrine therapy versus endocrine therapy alone was 0.573 (95% confidence interval [CI] 0.437–0.751) in premenopausal women and 0.798 (95% CI 0.644–0.990) in postmenopausal women.
Recently, abemaciclib received a positive opinion from the European Medicines Agency (EMA) for an extension to the existing indication on the basis of the positive results of the monarchE trial (Ann Oncol. 2021;32:1571–1581).
Lambertini notes, “The consistent effect, regardless of menopausal status, is reassuring. For years, we have thought that younger premenopausal patients had worse outcomes than older postmenopausal patients, but the current availability of new effective anticancer therapies is questioning this concept. At the time monarchE was conducted, a gonadotropin-releasing hormone (GnRH) agonist was not routinely used for ovarian function suppression as it is currently. I suspect that the numerically greater effect size observed with abemaciclib plus endocrine therapy in premenopausal versus postmenopausal women may be smaller now that GnRH agonists are the standard of care in such a high-risk patient population, but still, the consistency of these subgroup data are positive.”
Lambertini concludes that conducting analyses such as these are critical for all emerging agents in breast cancer beyond CDK4/6 inhibitors, including checkpoint inhibitors and PARP inhibitors, to better inform clinicians and patients on both their efficacy and safety as far as possible when treating younger women with breast cancer.
Abstracts discussed:
Furlanetto J, et al. Ovarian function in young patients (pts) treated with postneoadjuvant palbociclib (PAL) and endocrine therapy (ET) for hormone receptor (HR)-positive, HER2-negative early breast cancer (BC): explorative analysis in Penelope-B. ESMO Breast Cancer 2022, Abstract 60MO
Mini Oral Session 1, 03.05.2022, h. 16:15 – 17:30, Frankfurt Hall
Paluch-Shimon S, et al. Efficacy and safety results by menopausal status in monarchE: Adjuvant abemaciclib combined with endocrine therapy in patients with HR+, HER2- high-risk early breast cancer. ESMO Breast Cancer 2022, Abstract 63P
Poster Display Session, 04.05.2022, h. 12:15 – 13:00, Exhibition area
Watch the sessions also on the Congress virtual platform.