Study data report promising responses and safety profiles in patients with heavily pre-treated malignancies
Encouraging data from two early-phase, first-in-human trials of novel antibody–drug conjugates (ADCs) in patients with advanced solid tumours were reported at ESMO Congress 2022. ADCs employ a ‘Trojan-horse’ approach in which an antibody directed against a tumour target is attached to a chemotherapy agent, allowing this to be specifically delivered to a tumour cell.
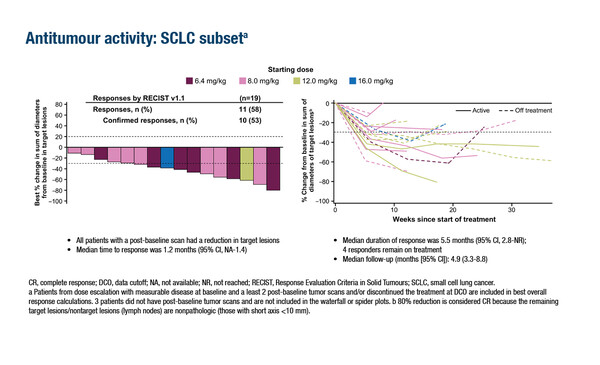
In the first presentation, follow-up data were reported from a phase I/II dose-finding study of DS-7300 (4.8 to 16.0 mg/kg) – a B7-H3-directed ADC with a topoisomerase I inhibitor payload (DxD) – in 147 patients with advanced solid tumours unselected for B7-H3 expression (Abstract 453O). In patients who had received a median of five prior lines of therapy, a response rate of 32% and a disease control rate (DCR) of 71.4% was observed. From 19 patients with small-cell lung cancer (SCLC), 11 (58%) patients achieved a partial or complete response, with a median duration of response of 5.5 months. No new safety signals were reported compared with earlier short-term data; however, more patients in the highest-dose cohort had grade ≥3 treatment-emergent adverse events (AEs) within a shorter median treatment duration than with lower doses.
Commenting on these data, Dr Andrew Furness from the Royal Marsden NHS Foundation Trust and Institute of Cancer Research, London, UK, notes, “Although the patient numbers in individual tumour types evaluated in this study are small, the greatest response is seen amongst those with SCLC. Whilst the duration of response in these patients might not be considered durable relative to other therapeutic modalities employed in solid tumours, these are certainly promising data, particularly as this is a very challenging disease type to treat in the refractory setting.”
A second presentation reported preliminary results from a phase I dose-escalation and dose-expansion study of the ADC zanidatamab zovodotin (ZW49), which comprises a bispecific HER2-directed antibody and a proprietary auristatin toxin (Abstract 460MO). In total, 77 patients with heavily pre-treated, HER2-positive cancers were evaluated, of whom 27% and 22% had gastroesophageal and breast cancers, respectively. Two dose-limiting toxicities (grade 2 keratitis >14 days) were observed in one patient on 1.75 mg/kg once weekly and one patient on 2.5 mg/kg once every 3 weeks. In total, 91% of patients had treatment-related AEs, which were mostly grade 1 or 2, with grade ≥3 treatment-related AEs reported in 12% of patients. Among 29 evaluable patients, the confirmed objective response rate was 31% and DCR was 72% with ZW49 2.5 mg/kg once every 3 weeks. “The safety signals in this trial look reasonable,” says Furness, “and additionally, there are early signs of antitumour activity across different tumour types, which would support further evaluation of this ADC.”
Remarking on these data overall, Furness says, “In my opinion there is great promise for ADCs, however the lack of durable disease control observed with standard systemic chemotherapy is also likely to apply here. I am looking forward to studies evaluating combination approaches, particularly those that seek to harness the power of the immune system. Further, given the well-recognised toxicity associated with standard chemotherapy approaches, I’m interested in whether ADCs might play an earlier role in cancers where (neo)adjuvant systemic therapy is indicated and may display a more favourable side-effect profile. Finally, it will be interesting to discover if next-generation ADCs can be developed that are conjugated to treatments other than chemotherapy, including those that serve to promote anti-cancer immunity or modulate the tumour microenvironment positively, leading either to direct anti-cancer activity or serving to condition the tumour microenvironment to better respond to approaches such as immune checkpoint inhibition or cell-based therapies.”
Abstracts presented:
Doi T, et al. DS-7300 (B7-H3 DXd antibody drug conjugate [ADC]) shows durable antitumor activity in advanced solid tumors: extended follow-up of a phase I/II study. ESMO Congress 2022, Abstract 453O
Proffered Paper Session: Developmental therapeutics, 10.09.2022, h. 10:15 – 11:45, Orléans Auditorium
Jhaveri K, et al. Preliminary results from a phase I study using the bispecific, human epidermal growth factor 2 (HER2)-targeting antibody-drug conjugate (ADC) zanidatamab zovodotin (ZW49) in solid cancers. ESMO Congress 2022, Abstract 460MO
Mini Oral Session, 12.09.2022, h. 16:30 – 18:00, Toulouse Auditorium