Two studies show that quality of life was maintained or improved with new treatments for advanced breast cancer
As presented at ESMO Breast Cancer 2022, analyses of health-related quality of life (QoL) measures from pivotal trials indicate favourable effects with trastuzumab deruxtecan in metastatic breast cancer and no detrimental effects with pembrolizumab in triple-negative breast cancer (TNBC).
Recently, it has been reported that trastuzumab deruxtecan reduced the risk of disease progression or death compared with trastuzumab emtansine in patients with HER2-positive metastatic breast cancer previously treated with trastuzumab and a taxane in the phase III DESTINY-Breast03 trial (N Engl J Med. 2022;386:1143–1154). At ESMO Breast Cancer 2022, results presented at a Proffered Paper Session demonstrate that trastuzumab deruxtecan was also associated with maintained or improved QoL versus trastuzumab emtansine (Abstract 163O).
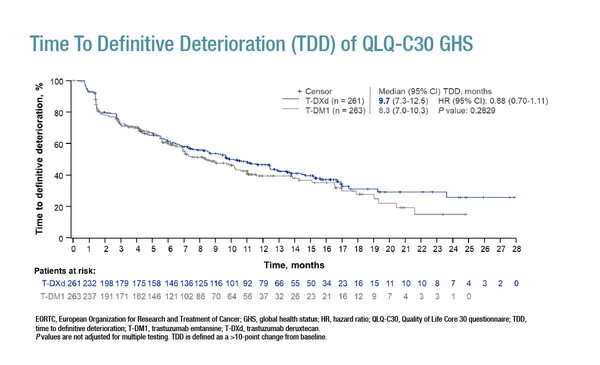
For global health status (GHS), assessed using the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire, change from baseline to the end of treatment was not meaningfully different (<10-point change) in both treatment groups. Median time to definitive deterioration (TDD) of QLQ-C30 GHS was 9.7 months for trastuzumab deruxtecan versus 8.3 months for trastuzumab emtansine (hazard ratio [HR] 0.88; 95% confidence interval [CI] 0.70–1.11; Figure) and longer TDD was observed with trastuzumab deruxtecan for all prespecified QLQ-C30 subscales, including emotional functioning (HR 0.69; 95% CI 0.53–0.89; p=0.0049) and pain (HR 0.75; 95% CI 0.59–0.95; p=0.0146). Using the EuroQol 5-dimension 5-level (EQ-5D-5L) visual analogue scale (VAS), median TDD was 13.2 months for trastuzumab deruxtecan versus 8.5 months for trastuzumab emtansine (HR 0.77; 95% CI 0.61–0.98; p=0.0354). A similar proportion of patients were hospitalised with trastuzumab deruxtecan (6.9%) and trastuzumab emtansine (7.2%), with longer median time to first hospitalisation observed with trastuzumab deruxtecan (219.5 versus 60.0 days).
“The DESTINY-Breast03 trial represents a breakthrough in terms of efficacy results, but a higher proportion of particular adverse events were seen with trastuzumab deruxtecan over trastuzumab emtansine, including pneumonitis, so it is important to complement survival data with information about the impact of this treatment on distinct domains of quality of life from the patient’s perspective,” notes Dr Ines Vaz-Luis from the Institut Gustave Roussy, Villejuif, France. “It is reassuring that trastuzumab deruxtecan did not have a detrimental effect overall, and in fact, important domains like emotional functioning and pain seem to have improved.”
In a second Proffered Paper presentation at ESMO Breast Cancer 2022, data from the KEYNOTE-355 trial showed QoL to be similar in patients with previously untreated, PD-L1–positive (combined positive score ≥10) advanced TNBC receiving pembrolizumab plus chemotherapy or placebo plus chemotherapy (Abstract 164O).
In the analysis, there were no between-group differences in change from baseline to week 15 in QLQ-C30 GHS, emotional functioning, physical functioning, or EQ-5D VAS scores. Similarly, there were no significant between-group differences in time to deterioration in QLQ-C30 GHS (HR 1.00; 95% CI 0.72–1.40), QLQ-C30 emotional functioning (HR 1.28; 95% CI 0.88–1.85) or QLQ-C30 physical functioning (HR 1.26; 95% CI 0.90–1.77). The KEYNOTE-355 trial had previously demonstrated an improvement in progression-free survival with the addition to chemotherapy of pembrolizumab versus placebo (Lancet. 2020;396:1817–1828).
“Adding pembrolizumab appears to have a neutral effect on QoL,” says Vaz-Luis. “Immunotherapy may be associated with side effects that we are just learning to deal with and it is encouraging that this population of patients did not perceive an adverse impact. Knowledge of the effects on QoL of agents with different mechanisms of action is becoming increasingly important as the breast cancer treatment landscape grows.”
Further discussing the future of patient-reported outcomes (PROs) assessment, she comments, “There is increasing use of PROs in trials, and over time, we are gaining a better understanding of how to collect and interpret these data. Typically, QoL instruments have been rather research focussed and not always patient-friendly. We now need to bring patients into the development and implementation of PROs to ensure these measures fulfil their purpose and fully reflect the patient experience. Moreover, we also need to better evaluate patient satisfaction with the particular regimen, and, specifically with new oral breast cancer agents, it will be important to accurately assess adherence.”
Abstracts discussed:
Curigliano G, et al. Patient-reported outcomes (PROs) from DESTINY-Breast03, a randomized phase 3 study of trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (pts) with HER2-positive (HER2+) metastatic breast cancer (MBC). ESMO Breast Cancer 2022, Abstract 163O
Proffered Paper Session 1, 03.05.2022, h. 13:45 – 15:15, Munich Hall
Cescon DW, et al. Health-related quality of life (HRQoL) with pembrolizumab (pembro) + chemotherapy (chemo) vs placebo (pbo) + chemo as 1L treatment for advanced triple-negative breast cancer (TNBC): Results from KEYNOTE-355. ESMO Breast Cancer 2022, Abstract 164O
Proffered Paper Session 1, 03.05.2022, h. 13:45 – 15:15, Munich Hall
Watch the sessions also on the Congress virtual platform.