A translational analysis of the DAISY trial improves knowledge on the mechanisms of action of the antibody–drug conjugate
Antibody–drug conjugates (ADCs) have transformed the field of breast oncology and the new-generation ADC, trastuzumab-deruxtecan (T-DXd), has shown remarkable efficacy in HER2+ metastatic breast cancer (N Engl J Med. 2022;386:1143–1154), with some activity even in low-HER2-expressing tumours (J Clin Oncol. 2020;38:1887–1896).
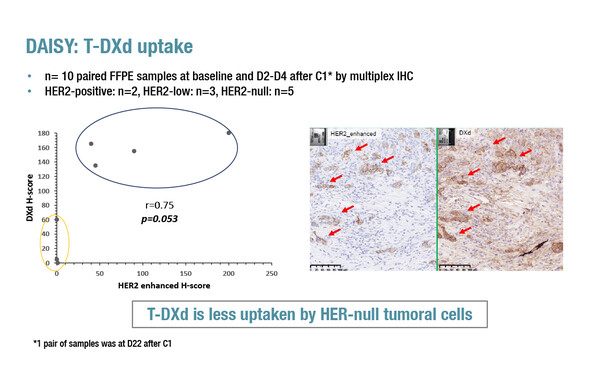
In a late-breaking presentation at ESMO Breast Cancer 2022, a significantly greater uptake of T-DXd in HER2-low cells than in HER2-negative cells (p=0.053) was reported by a translational analysis of the DAISY trial assessing the ADC efficacy in 179 patients with metastatic breast cancer according to HER2 expression status (Abstract LBA1). Efficacy data presented last year, in terms of response rates and progression-free survival (PFS), were associated with the degree of HER2 expression, being highest in patients with HER2-positive disease, lower in those with HER2-low disease and lower again in patients with HER2-negative disease (San Antonio Breast Cancer Symposium 2021).
In the analysis, data were collected on biopsies from 10 patients. According to clustering algorithms, there was a cluster with a lower best overall response that had a high percentage of HER2-negative cells. Treatment with T-DXd did not lead to modulation of T cells or macrophages but there was a reduction in PD-L1 expression in HER2-positive disease (p=0.02). At progression, 13/20 (65%) of patients had a reduction in HER2 expression.
Associate Professor Aditya Bardia, from Massachusetts General Hospital, Boston, MA, USA, says that there are two particularly striking findings from this translational analysis. “The first is that the low uptake of T-DXd in the HER2-negative cells strongly suggests that the activity of T-DXd in these cells is due to the bystander effect, whereby the payload delivered to tumour cells diffuses across cell membranes into neighbouring tumour cells. The second is that activity of T-DXd was also related to the spatial distribution of HER2; if HER2-expressing cells were spatially distant, the response rate was low, but if they were clustered closer, the response was higher. So the study provides important evidence that response to treatment is not influenced solely by the presence or absence of the HER2 antigen, but also by its spatial distribution within the tumour.” Bardia thinks that these results will motivate researchers to look more closely at the spatial distribution of antigens within tumours and suggests that molecular analyses of tissue from clinical trials – retrospective and prospective – will help to determine the influence of this factor on response to treatment.
The use of immune checkpoint inhibitors to enhance the efficacy of T-Dxd is being investigated in early-phase studies (ESMO Open. 2021;6:100204). In a second oral presentation at ESMO Breast Cancer 2022, the primary analysis of part 2 of a two-part, phase Ib DS8201-A-U105 trial (NCT03523572) reported that adding nivolumab to T-DXd did not improve efficacy compared with that expected for T-DXd monotherapy in patients with HER2-expressing metastatic breast cancer (Abstract 162O). Among 48 immunotherapy-naïve patients, 32 were HER2-positive (after T-DM1) and 16 were HER2-low (after standard of care). Confirmed objective response rates were 65.6% and 50.0% for HER2-positive and HER2-low patients, respectively. The median duration of response was not evaluable for HER2-positive patients and was 5.5 months for HER2-low patients. Median PFS times for HER2-positive and HER2-low patients were 11.6 months and 7.0 months, respectively. Treatment-emergent adverse events grade ≥3 were seen in 50.0% of patients and 14.6% of patients had drug-related interstitial lung disease.
“We need to be careful not to overdraw conclusions, particularly comparative efficacy, from a phase I trial. The jury is still out regarding role of immunotherapy with T-DXd," says Bardia. “Further research is needed to understand the role of different payloads in mediating neoantigen generation and immune triggers to develop rationale immunotherapy combinations with ADCs.”
Abstracts discussed:
Mosele MF, et al. Unraveling the mechanism of action and resistance to trastuzumab deruxtecan (T-DXd): biomarker analyses from patients from DAISY trial. ESMO Breast Cancer 2022, Abstract LBA1
Proffered Paper session 2, 04.05.2022, h. 16:45 – 18:15, Berlin Hall
Hamilton EP, et al. Primary analysis from DS8201-A-U105: A 2-part, open label, phase 1b trial assessing trastuzumab deruxtecan (T-DXd) with nivolumab (nivo) in patients (pts) with HER2-expressing advanced breast cancer. ESMO Breast Cancer 2022, Abstract 162O
Proffered Paper Session 1, 03.05.2022, h. 13:45 – 15:15, Munich Hall
Watch the sessions also on the Congress virtual platform.