Novel scheduling of RP-3500 mitigates haematological toxicity and may facilitate combination therapy but tumour types most likely to respond remain to be defined
A new-generation ataxia telangiectasia and Rad3-related (ATR) inhibitor – the highly selective RP-3500 – is being investigated in the phase I/IIa TRESR trial (NCT04497116) in patients with solid tumours and ATR inhibitor-sensitising mutations. Following on from last year’s preliminary findings of antitumour activity in the phase I part of the trial (Mol Cancer Ther. 2021;20(12 Supplement):Abstract CC04-01), results from dose-scheduling and pharmacokinetic (PK) analyses presented at the ESMO Targeted Anticancer Therapies Congress 2022 give further insights into the clinical potential of this agent.
ATR kinase is an attractive target for anticancer therapies, playing a vital role in co-ordinating the cell response to DNA damage and replication stress. However, while ATR inhibitors with utility in the clinical setting have remained largely elusive, the introduction of newer generation agents has come with some promise in early-phase clinical trials (Curr Res Pharmacol Drug Discov. 2021;2:100017).
“We have powerful, specific ATR inhibitors that should be beneficial in a treatment setting, but we are struggling to deliver them in a way that minimises the risks of haematological toxicities without compromising efficacy,” explains Dr Joaquin Mateo from the Vall d'Hebron Institute of Oncology (VHIO), Barcelona, Spain. Commenting on the importance of the TRESR trial in determining ATR inhibitor utility, Mateo, who was not involved in the trial or in the analyses presented at the Congress, says: “Most phase I trials select a recommended phase II dose (RP2D) based primarily on dose-limiting toxicities encountered during cycle 1 of therapy. However, we know that ATR-related haematological toxicities can appear later in the course of therapy.”
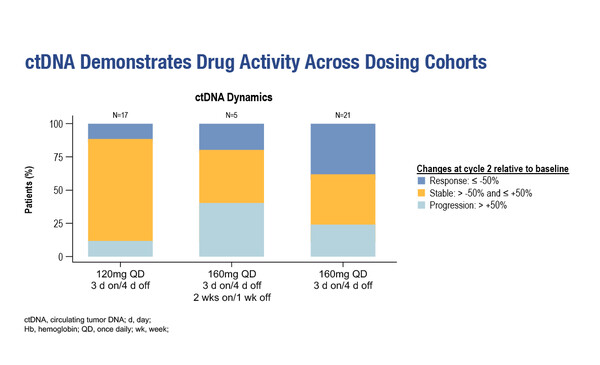
The TRESR trial uses a novel design to enable such later-emerging adverse events to be taken into account (Abstract 5MO). Bayesian optimal interval-based dose escalation was based on preclinical PK/pharmacodynamic models, clinical PK, biomarkers, circulating tumour (ct)DNA and safety data, leading to the adoption of the intermittent ‘3 days on/4 days off’ administration schedule for RP-3500. A nomogram to mitigate anaemia was developed using haematological side-effects emerging in treatment cycle 1. Long-term safety was assessed in a subset of patients with solid tumours from three dose-expansion cohorts with 3 days on/4 days off administration that was either continuous every week or administered 2 weeks on/1 week off (120 mg once daily, continuous [n=25]; 160 mg once daily, continuous [n=34]; 160 mg once daily, 2 weeks on/1 week off [n=26]). Across dosing cohorts, grade 3 anaemia was the most common toxicity and a decrease in circulating tumour (ct)DNA mean variant allele frequency in cycle 2 indicated RP-3500 activity. The data support the use of the 160 mg once-daily, continuous dose of RP-3500 in ongoing studies. “The importance of the data is two-fold,” says Mateo. “Firstly, it improves understanding of how the delivery of RP-3500 can be optimised in the clinical setting, particularly to allow its combination with treatments with overlapping toxicities, such as PARP inhibitors, which is probably how ATR inhibitors could make the biggest difference for patients. More generally, the data propose an important consideration for the design of future phase I clinical trials.”
Additional data from TRESR are given in two poster presentations at the Congress, which inform the dosing and safety of RP-3500. In one presentation, population PK data – based on 121 patients receiving RP-3500 at 5–200 mg once or twice daily, according to two different schedules: 5 days on/2 days off and 3 days on/4 days off – confirm its predictable PK profile, with a trend for renal clearance to impact the clearance of RP-3500. An initial exposure–response assessment revealed no treatment-related changes in QT interval beyond 4 hours post-dose at cycle 1/day 1 and the investigators concluded that RP-3500 at therapeutically relevant doses posed a very low risk of clinically impacting QT (Abstract 9P). In a food-effect investigation in 12 patients, although administration of RP-3500 with a high-fat/high calorie meal delayed median Tmax by 3 hours and reduced mean Cmax by 45% and AUC0–24 by 16%, compared with the fasted state, plasma levels of RP-3500 required for pharmacological activity showed no marked difference between the fed and fasted states. The recommended phase II dose and schedule was established as 160 mg daily on a 3 days on/4 days off schedule (Abstract 8P).
With the clinical potential of ATR inhibitors looking closer to a clinical reality, the tumour types most likely to benefit need to be defined. “The spotlight so far has been on tumours harbouring ATM loss, because the concomitant loss of ATR and ATM could be lethal to cancer cells,” Mateo concludes. “The TRESR investigators have looked beyond this and, building on additional preclinical findings, are investigating activity in tumours with alterations in a number of other genes involved in DNA damage response. Particularly interesting is the focus on patients with BRCA1/2 mutations who have already progressed on a PARP inhibitor; ATR inhibition may drive tumour cell killing by increasing replication stress in these tumours that have incompletely recovered homologous recombination repair.”
Abstracts discussed:
Fontana E, et al. Comprehensive dose-finding strategy for single-agent RP-3500, a highly selective inhibitor of ataxia telangiectasia and Rad3-related (ATR) kinase. ESMO Targeted Anticancer Therapies Congress 2022, Abstract 5MO
Mini Oral Session, 07.03.2022, h. 17:15 – 18:20, Channel 1
Lee E, et al. Preliminary population pharmacokinetic (PopPK) co-variates and exposure response (ER) assessment of QT for RP-3500, a highly potent and specific inhibitor of ataxia telangiectasia and Rad3-related (ATR) protein kinase. ESMO Targeted Anticancer Therapies Congress 2022, Abstract 9P
Lheureux S, et al. Pharmacokinetic (PK) profile and food effect of RP-3500, a highly potent and specific inhibitor of ataxia telangiectasia and Rad3-related (ATR) protein kinase in patients (pts) with cancer. ESMO Targeted Anticancer Therapies Congress 2022, Abstract 8P