Promising new data are presented for nivolumab plus ipilimumab and the single tremelimumab regular interval durvalumab regimen in Asian patients and those with impaired liver function
Data from two phase III studies presented at the ESMO Asia Congress 2024 (Singapore, 6–8 December) help to further establish the place of immunotherapy combinations in the first-line treatment of unresectable hepatocellular carcinoma (uHCC).
Results in Asian patients were consistent with those in the global population in an interim analysis of the CheckMate 9DW trial comparing first-line nivolumab plus ipilimumab versus lenvatinib or sorafenib in uHCC (Abstract 126O). Median overall survival (OS) was 34.0 months with nivolumab plus ipilimumab versus 22.5 months with lenvatinib or sorafenib (hazard ratio [HR] 0.75; 95% confidence interval [CI] 0.54–1.03) in 280 Asian patients at a median follow-up of 35.7 months. In the 668 patients randomised globally, the HR was similar at 0.79 (95% CI 0.65–0.96; p=0.0180). In Asian patients, the objective response rate was 37% with nivolumab plus ipilimumab versus 14% with lenvatinib or sorafenib (global population: 36% versus 13%, respectively). The median duration of response was not reached with nivolumab plus ipilimumab versus 18.5 months with lenvatinib or sorafenib (global population: 30.4 months versus 12.9 months, respectively). Grade 3–4 treatment-related adverse events occurred in 43% of Asian patients receiving nivolumab plus ipilimumab and 45% receiving lenvatinib or sorafenib, with no unexpected safety concerns.
“The first overall data from the interim analysis of the CheckMate 9DW trial were convincing, with a high response rate and very good median OS (J Clin Oncol. 2024;42(17 Suppl):LBA4008). Asian patients tend to respond a little better in uHCC trials, but it is reassuring to see there are no major discrepancies compared with the global population,” says Prof. Arndt Vogel from Hannover Medical School, Germany and the University of Toronto, Canada, who also cautions that toxicity would have to be managed carefully in all patients in the clinic. “Although nivolumab plus ipilimumab is unlikely to be the only standard of care for all patients in the first-line treatment of uHCC, it will be an excellent option for some patients. We definitely need to develop biomarkers to better select patients for the different treatment options we have today for systemic therapies in HCC,” he notes.
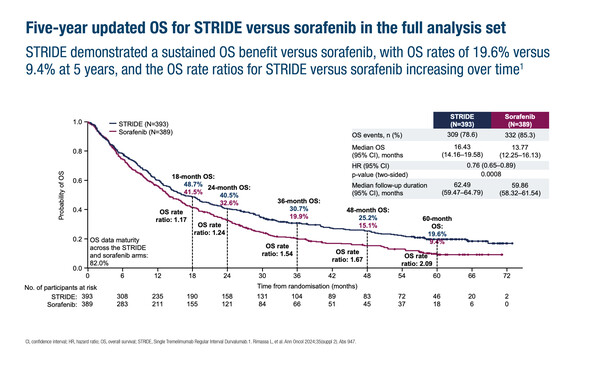
Impressive 5-year OS data from the HIMALAYA study evaluating the single tremelimumab regular interval durvalumab (STRIDE) regimen in patients with uHCC (Abstract 127O), extend previously published 4-year results (Ann Oncol. 2024;35:448–457). The new analysis demonstrated 5-year OS rates of 19.6% with STRIDE versus 9.4% with sorafenib (HR for median OS of 0.76; 95% CI 0.65–0.89). In a third arm, the 5-year OS rate was 14.4% with durvalumab monotherapy.
In addition, when OS was assessed by baseline liver function, the researchers found long-term OS benefit for STRIDE over sorafenib regardless of albumin-bilirubin grade. In both subgroups analysed – patients with albumin-bilirubin grade 1 and patients with albumin-bilirubin grade 2–3 – the HR for OS was 0.79 for STRIDE versus sorafenib. The rate of treatment-related serious adverse events was 17.5% with STRIDE in all patients and appeared similar in both albumin-bilirubin subgroups (grade 1: 20.4%; grade 2–3: 14.0%).
“Over the last few years, significantly longer median OS with immunotherapy showed that progress was being made. But now, as observed in the HIMALAYA trial, improvements are clearly being seen at the tail end of the curve. One in five patients was alive after 5 years with STRIDE treatment indicating that this combination is working beyond the initial phase of tumour control. Rarely, if at all, are 5-year OS data presented in uHCC and so this demonstration of long-term benefit is very exciting,” says Vogel. He notes that patients with good liver function tend to respond better in trials than those with impaired function but thinks the similar efficacy with STRIDE regardless of liver function will support its use in clinical practice.
Vogel concludes, “Bispecific antibodies targeting dual inhibitory checkpoints and also the development of novel immunotherapy targets, such as TIGIT, are being evaluated in early-phase trials in liver cancer, with the hope of continued progress to improve long-term survival.”
Programme details
Yau T, et al. Nivolumab (NIVO) plus ipilimumab (IPI) vs lenvatinib (LEN) or sorafenib (SOR) as first-line (1L) treatment for unresectable hepatocellular carcinoma (uHCC): CheckMate 9DW Asian subgroup analysis. ESMO Asia Congress 2024, Abstract 126O
Proffered Paper Session – Gastrointestinal Tumours, 06.12.2024, h. 13:45 – 15:15, Hall 406
Kudo M, et al. Five-year overall survival (OS) and OS by baseline liver function from the phase 3 HIMALAYA study of tremelimumab (T) plus durvalumab (D) in unresectable hepatocellular carcinoma (uHCC). ESMO Asia Congress 2024, Abstract 127O
Proffered Paper Session – Gastrointestinal Tumours, 06.12.2024, h. 13:45 – 15:15, Hall 406