Safety and efficacy data of some novel immunotherapy agents are encouraging, but more research is needed to identify biomarkers and the optimal treatment schedule
T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif (ITIM) domains (TIGIT) and stimulation of interferon genes (STING) are signalling pathways that have recently emerged as potential immunotherapy targets in cancer following promising results in transplantable cancer mouse models. Preliminary data from several studies investigating the safety and efficacy of novel agents targeting these pathways were presented at the ESMO Congress 2023 (Madrid, 20–24 October), showing some early promise. “TIGIT plays an important role in regulating immune responses at the immune synapses of T and NK cells, and cyclic GMP-AMP synthase (cGAS)-STING pathway functions as a microbial-sensing defence mechanism against infections but may also underpin natural antitumour immune responses, making both potentially very important targets in cancer immunotherapy,” explains Dr Ignacio Melero from Universidad de Navarra, Pamplona, Spain. “The activation of the STING pathway is gaining the attention of researchers after robust preclinical data in mouse tumour models. Pioneering work by Tom Gajewski’s group created much enthusiasm and various companies undertook the generation of STING agonists to be delivered intratumourally or systemically. Unfortunately, for the time being, evidence of clinical activity is very limited.”
Promising data were reported for the anti-TIGIT antibody tiragolumab combined with the PD-L1 inhibitor atezolizumab in a phase II study in PD-L1-selected, recurrent or metastatic non-small-cell lung cancer (NSCLC) (Lancet Oncol. 2022;23:781–792). In extensive SCLC, phase III data have not rendered evidence for an additional progression-free survival benefit in extensive SCLC (J Clin Oncol. 2022;40(17_suppl):LBA8507). In a Mini Oral Session, the safety and efficacy data were presented for etigilimab (an anti-TIGIT monoclonal antibody) plus the PD-1 inhibitor nivolumab from the open-label phase Ib/II ACTIVATE study of patients with advanced or metastatic solid tumours with no curative or standard of care therapies (Abstract 1021MO). The analysis included selected patients with tumour types not typically responsive to anti-PD-(L)1 monotherapy, including gynaecological malignancies. Of 40 evaluable patients, three (7.5%) achieved a complete response, seven (17.5%) had a partial response, 11 (27.5%) had stable disease and 19 (47.5%) experienced disease progression. In the safety evaluable cohort (n=76), the combination of etigilimab and nivolumab was found to be safe and well tolerated. Commenting on the results, Melero says, “Some promising clinical responses were observed in this study, particularly in cases of carcinoma of the cervix, where three complete responses were attained. It will be interesting to see whether these responses are primarily solely due to the PD-1 inhibitor or whether they can be attributed to the combination of these two agents. An expansion study seems to be warranted in patients suffering with this malignancy.”
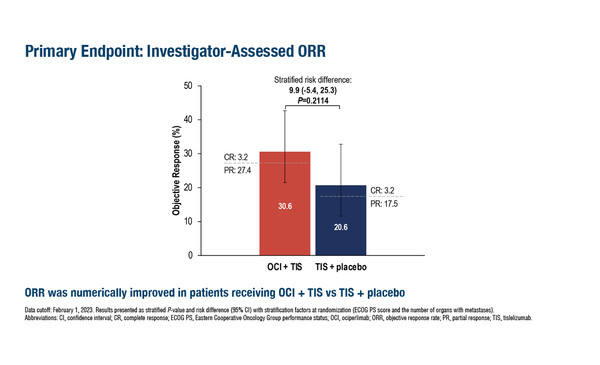
In a second study, the phase II AdvanTIG-203, efficacy and safety data were promising for the combination of anti-TIGIT monoclonal antibody ociperlimab plus the PD-1 inhibitor tislelizumab in patients with unresectable, locally advanced, recurrent or metastatic oesophageal squamous cell carcinoma showing a PD-L1 tumour area positivity ≥10% (Abstract 1020MO). Among 125 patients randomised, progression-free survival was comparable between the ociperlimab plus tislelizumab arm and the tislelizumab-only arm (hazard ratio [HR] 1.01, 95% confidence interval [CI] 0.64–1.59), but a trend towards an improved objective response rate was observed for the ociperlimab-treated group (32.3% [95% CI 20.9–45.3] versus 25.4% [95% CI 15.3–37.9]). Melero notes, “This is a disease in which checkpoint inhibitors are known to have activity; however, it appears that the clinical responses here cannot be fully attributed to tislelizumab and that some of the clinical efficacy may be due to the addition of ociperlimab.” He cautions, “However, the caveat to this study is that it has focused on patients with a PD-L1 positive tumour and this may bias the results towards greater efficacy.”
Preliminary data from two phase I studies involving novel STING agonists were presented in poster sessions. Firstly, data on dazostinag, either alone or in combination with the PD-1 inhibitor pembrolizumab, were reported for patients (n=79) with advanced or metastatic solid tumours (Abstract 1029P). Dazostinag displayed linear pharmacokinetics across the dose range, unaffected by the addition of pembrolizumab. As a single agent, dazostinag achieved a >3.5-fold induction of a 24-gene STING signature score. Moreover, at a dose of 5 mg, with or without pembrolizumab, dazostinag induced a >2-fold increase in cytokines, including interferon-gamma (IFN-γ) and IFN-γ-inducible protein 10 (IP-10), and increased proliferation of peripheral Ki67+CD8+ T cells. Three clinical responses were also confirmed in patients treated with dazostinag plus pembrolizumab. Melero comments, “This agent is interesting as it can be administered intravenously, reportedly without the dose-limiting toxicities observed when other STING agonists are given intravenously.” The second poster (Abstract 1030P) presented data on the intratumoural STING agonist BI 1387446, used alone or in combination with the PD-1 inhibitor ezabenlimab, in patients (n=41) with advanced or metastatic solid tumours. The maximum tolerated dose could not be determined and BI 1387446 was well tolerated as monotherapy or in combination with ezabenlimab. The best response in this study was stable disease, observed in 46.2% and 53.3% of patients receiving BI 1387446 as monotherapy or combination therapy, respectively. Commenting on these findings, Melero highlights that repeated intratumoural injections of this therapy appear to be well tolerated, with only minor side effects reported.
Melero concludes, “The data reported here for these novel agents are promising, but there is a need to identify predictive biomarkers that may allow us to determine the likelihood of clinical activity in patients receiving agents acting on either the TIGIT or STING pathway. Furthermore, we need to empirically determine the most efficacious drug combinations to use with these agents.”
Abstracts discussed:
Xu R-H, et al. AdvanTIG-203: Phase 2 randomized, multicenter study of ociperlimab (OCI) + tislelizumab (TIS) in patients (pts) with unresectable, locally advanced, recurrent/metastatic esophageal squamous cell carcinoma (ESCC) and programmed cell death-ligand 1 (PD-L1) positivity. ESMO Congress 2023, Abstract 1020MO
Mini Oral Session – Investigational immunotherapy, 21.10.2023, h. 16.30 – 17.50, Málaga Auditorium – Hall 10
Mckean M, et al. Safety and efficacy of etigilimab (anti-TIGIT) with nivolumab (anti-PD1) in recurrent/advanced solid tumors. ESMO Congress 2023, Abstract 1021MO
Mini Oral Session – Investigational immunotherapy, 21.10.2023, h. 16.30 – 17.50, Málaga Auditorium – Hall 10
Olszanski AJ, et al. Dazostinag (TAK-676) alone and in combination with pembrolizumab (pembro) in patients (pts) with advanced or metastatic solid tumors: preliminary safety, PK/PD, and anti-tumor activity in a phase 1 dose escalation study supporting a recommended dose for expansion (RDE). ESMO Congress 2023, Abstract 1029P
Poster Display – Investigational immunotherapy, 23.10.2023, h. 09.00 – 17.00, Hall 8
Calvo E, et al. Phase I, first-in-human trial evaluating the STING agonist BI 1387446 alone and in combination with ezabenlimab in solid tumors. ESMO Congress 2023, Abstract 1030P
Poster Display – Investigational immunotherapy, 23.10.2023, h. 09.00 – 17.00, Hall 8