In the Cohort B of the TACTI-003 study, the combination therapy led to clinically meaningful response rates in patients with recurrent or metastatic disease
Data presented at the ESMO Virtual Plenary in July 2024 confirm the antitumour activity of the combination of eftilagimod alpha, a soluble LAG-3 protein and major histocompatibility complex (MHC) class II agonist, and the anti-PD-1 pembrolizumab as first line treatment in patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) and negative PD-L1 expression (CPS<1, Cohort B) (VP5-2024).
In the TACTI-003 study – a multicentre, randomised, open-label Phase IIb trial – 31 patients with hypopharynx (3%), larynx (32%), oral cavity (29%) and oropharynx (36%) tumours were treated with subcutaneous eftilagimod (30 mg) every two or three weeks and intravenous pembrolizumab (400 mg) every six weeks for up to 24 months.
Final results show that the combination therapy led to a clinically meaningful objective response rate (ORR) of 35.5% (95% CI: 19.2-54.6) and disease control rate (DCR) was 58.1%, including nearly 10% of complete responses, in the PD-L1 CPS<1 population. Responses were observed regardless of human papillomavirus (HPV) status.
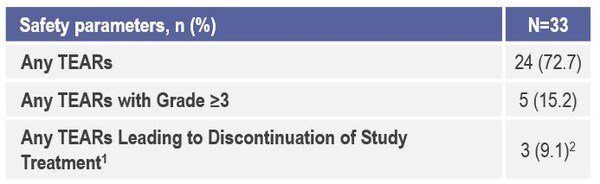
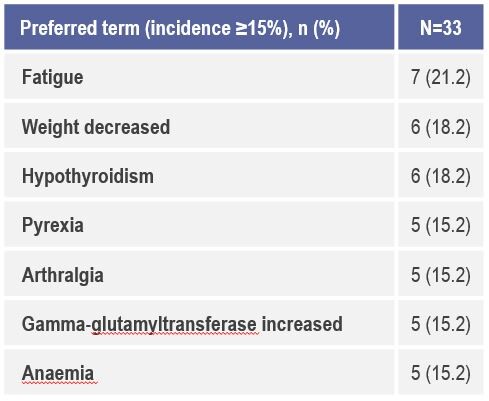
The study also confirmed eaftilagimod alpha is well tolerated and the does not appear to add significant toxicity nor an increase in immune-mediated adverse events when compared to historical data (Figure 1 and 2). “The results presented are encouraging in terms of antitumour activity and response rates, but in order to be clinically relevant in the first-line setting they should be coupled with a significant survival benefit, and a phase three randomised study comparing the combination therapy to the current standard of care should be now pursued,” highlights Dr Marc Oliva, Catalan Institute of Oncology, Barcelona, Spain, commenting on the data.
Previous studies reported an encouraging efficacy of eftilagimod alpha in combination with pembrolizumab in HNSCC patients after progressing on first-line chemotherapy (J Clin Oncol 2023; 41, 6029-6029) and as frontline treatment in non-small cell lung cancer (NSCLC) (Ann of Oncol 2023; 34, S755-S851).
“LAG-3 is one of the most promising immuno-oncology therapeutic targets across various tumour types including HNSCC. Anti-LAG-3 agents are already being tested in phase 3 randomised clinical trials for first line recurrent/metastatic HNSCC in combination with anti-PD-1 with or without the addition of other immune-checkpoint inhibitors”, concludes Oliva. “However, as the field moves towards personalised medicine, biomarker development is crucial to identify patients who will really benefit from LAG-3 targeting agents.”