Two studies report the results about the use of trastuzumab deruxtecan and androgen deprivation therapy for patients with this rare cancer
Encouraging results from a study with an antibody–drug conjugate (ADC) and negative findings from another study with an androgen deprivation therapy (ADT) presented at the ESMO Congress 2024 (Barcelona, 13–17 September) show that treatments already in use for other cancers may become options for recurrent/metastatic salivary gland cancers (SGCs) and highlight the potential opportunities to tailor therapy for this heterogenous, rare disease.
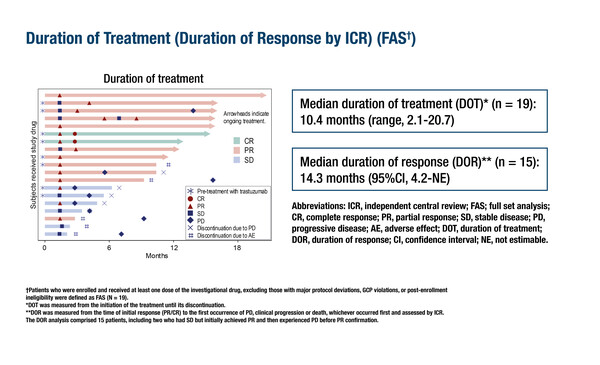
Results presented from MYTHOS, an investigator-initiated phase II, multicentre, open-label, single-arm study of the ADC trastuzumab deruxtecan conducted in Japan, focused on a cohort of 19 patients with HER2-expressing (immunohistochemistry 3+ or 2+/in-situ hybridization-positive) recurrent/metastatic SGC (Abstract 849O). At a median follow-up of 15.0 months, the independent central review-assessed objective response rate (ORR) was 68.4% and the median progression-free survival (PFS) was 15.9 months. The median duration of response was 14.3 months in 15 assessed patients. The safety data were in line with the known profile of trastuzumab deruxtecan, except for interstitial lung disease toxicity that was observed in 36.8% of patients, with 5.3% considered severe (grade 3).
Commenting on the study findings, Dr Anna Spreafico from the Princess Margaret Cancer Centre and University of Toronto, Canada reflects, “The HER2-positive cohort results align well with the very good response rate reported recently in the much larger DESTINY-PanTumor02 trial (J Clin Oncol. 2024;42:47–58) and affirm the potential role of this agent in salivary gland tumours, a rare disease for which treatment options are limited. It will be very interesting to see the results from the HER2-low cohort once these are available, as trastuzumab deruxtecan might have some efficacy in this group, where there is also a large unmet treatment need.”
The second phase II study comprised a randomised cohort of 54 untreated patients who received either ADT or chemotherapy, and an untreated cohort of pre-treated patients who received ADT (LBA36). The majority of patients (66.7%) had androgen-receptor (AR)-positive salivary duct cancer and a minority (33.3%) had adenocarcinoma not otherwise specified. The results presented indicated that ADT did not show superiority nor non-inferiority to chemotherapy, with median PFS of 4.0 months in the randomised ADT arm and 6.5 months in the chemotherapy arm. In the pre-treated cohort, the confirmed ORR was 19.6% with one complete response. Reflecting on these results, Spreafico suggests, “This is the first randomised study to evaluate the role of ADT as compared to chemotherapy in this patient population and several patients respond to ADT. Identifying biomarkers for improving patient selection is crucial at this juncture to provide appropriate treatment and spare additional toxicities.”
Guidelines support tumour profiling with molecular testing and immunohistochemistry to guide treatment selection in recurrent/metastatic SGCs (ESMO Open. 2022;7:100602). “The investigators of these two studies demonstrated that tumour profiling can guide the use of treatments effective in other indications, deepening our knowledge of this rare disease and offering patients more personalised treatment options,” concludes Spreafico.
Programme details
Kinoshita I, et al. Phase II study of trastuzumab deruxtecan in patients with HER2-positive recurrent/metastatic salivary gland cancer: results from the MYTHOS trial. ESMO Congress 2024, Abstract 849O
Proffered Paper Session – Head and neck cancer, 15.09.2024, h. 10:15 – 11:45, Valencia Auditorium – Hall 5
Licitra LFL, et al. A randomised phase II study to evaluate the efficacy and safety of androgen deprivation therapy (ADT) vs chemotherapy (CT) in patients with recurrent and/or metastatic, androgen receptor (AR) expressing, salivary gland cancers. ESMO Congress 2024, LBA36
Proffered Paper Session – Head and neck cancer, 15.09.2024, h. 10:15 – 11:45, Valencia Auditorium – Hall 5