The Pan-Asian adaptation of the EHNS-ESMO-ESTRO Clinical Practice Guidelines is presented at the ESMO Asia Virtual Oncology Week 2021
In 2020, guidelines on squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx were published by the European Head and Neck Society (EHNS), the European Society for Medical Oncology (ESMO) and the European SocieTy for Radiotherapy and Oncology (ESTRO) (Ann Oncol. 2020;31:1462‒1475).
“Many of the 32 EHNS-ESMO-ESTRO recommendations are relevant to all populations with squamous cell carcinomas of the head and neck (SCCHN), but we felt that there were some areas that needed to be modified to reflect the Asian-specific situation,” explains Dr Bhumsuk Keam, Seoul National University Hospital, South Korea, first author of the Pan-Asian adaptation of the EHNS-ESMO-ESTRO Clinical Practice Guideline presented at the ESMO Asia Virtual Oncology Week 2021. After a short delay due to the COVID-19 pandemic, ESMO and the Korean Society of Medical Oncology (KSMO) convened a virtual meeting with six other Asian oncological societies to discuss adaptations necessary to help the guidelines better meet the needs of Asian patients.
As an example of the changes made in the Pan-Asian adaptation, a consensus was reached on considering a hypoxic radiosensitiser, only if available, to increase locoregional control and disease-free survival compared with radiotherapy alone. This was due to the fact that hypoxic radiosensitisers are either not widely used or are not approved in some Asian countries.
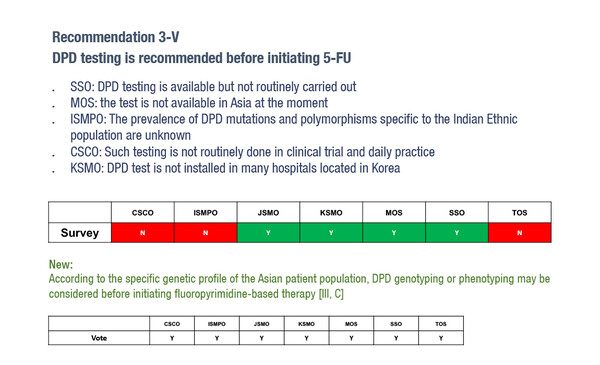
Also, reflecting genetic differences of populations in different geographical areas, dihydropyrimidine dehydrogenase deficiency (DPD) testing prior to 5-fluorouracil (5-FU) initiation is not routinely performed in Asian countries due to its low frequency in the Asian population. This is captured in the Pan-Asian adaptation, which states, ‘According to the specific genetic profile of the Asian patient population, DPD genotyping or phenotyping may be considered before initiating fluoropyrimidine-based therapy’.
Regarding treatment, extra text has been added to acknowledge that weekly cisplatin 40 mg/m2 is an alternative option to 3-weekly cisplatin 100 mg/m2 in the post-operative setting in Asian countries. In addition, for patients with recurrent/metastatic SCCHN, there was much discussion about the PD-L1 combined positive score (CPS) as an indicator of response to pembrolizumab based on data from the phase III KEYNOTE-048 trial (Lancet. 2019;394:1915‒1928). While the EHNS-ESMO-ESTRO recommendation for patients with CPS ≥1 states ‘chemotherapy plus pembrolizumab is recommended when rapid tumour shrinkage is needed’, the Pan-Asian adaptation highlights that ‘the choice of pembrolizumab monotherapy or chemotherapy plus pembrolizumab may be based on CPS, tumour burden, and symptoms’.
For patients with recurrent/metastatic SCCHN not expressing PD-L1, platinum/5-FU/cetuximab remains the standard therapy. However, in addition to docetaxel, cisplatin and cetuximab (TPeX), the Pan-Asian adaptation also includes pembrolizumab plus chemotherapy and paclitaxel plus carboplatin plus cetuximab as treatment options.
Keam highlights that one of the key benefits of the Pan-Asian adaptations of ESMO Clinical Practice Guidelines (PAGA) is that they can be used for advocacy. “Experts in some Asian countries have to debate the benefits of novel agents with the regulatory authorities to gain approval. The existence of the PAGA means there is even more evidence ‒ focussed on the specific situations in our countries ‒ that can be used to push for policy changes and improve access.” He adds, “The adaptation process has also helped me to recognise just how important active communication is between Asian countries in terms of improving the quality of the treatment ‒ we need even more collaboration.”
The PAGA project was initiated in 2016 with the aim of tailoring ESMO Clinical Practice Guideline recommendations to ensure that they incorporate ethnic, scientific, socioeconomic and local practice elements to meet the needs of cancer patients in Asia. Building on successful collaborations with key opinion leaders from seven partner oncology societies in China, India, Japan, South Korea, Malaysia, Singapore and Taiwan, ESMO is now expanding the number of partners to ten, with the addition of societies from the Philippines, Indonesia and Thailand. On occasion of the ESMO Asia Virtual Oncology Week 2021, a dedicated web page has been launched where users can find all Pan-Asian Guidelines Adaptations produced so far in one single place
Keam B. Head & neck: Presentation of the Pan-Asian adapted ESMO Clinical Practice Guideline recommendations. ESMO Asia Virtual Oncology Week 2021
Pan-Asian adapted ESMO Clinical Practice Guidelines lecture on head & neck cancer. ESMO Asia Virtual Oncology Week 2021, 21.11.21, h. 16:30 – 16:50 (Singapore time), Channel 1