In an early-phase study, the selective inhibitor nanatinostat was well tolerated with evidence of antitumour activity in combination with antiviral therapy
Early results with the targeted agent nanatinostat in patients with recurrent/metastatic Epstein-Barr virus (EBV)-positive nasopharyngeal carcinoma (NPC) were presented at the ESMO Asia Congress 2023 (Singapore, 1–3 December) (Abstract 354O).
Nanatinostat is a class-I selective oral histone deacetylase inhibitor that activates the EBV lytic cycle and makes EBV-positive tumour cells vulnerable to antivirals. It has shown promising safety and efficacy in combination with the ganciclovir prodrug, valganciclovir, in recurrent EBV-positive lymphoid malignancies (Blood Adv. 2023;7:6339–6350). NPC has the highest incidence rates in the Asian region and although research progress has increased treatment options for patients in the last three decades, effective agents targeting EBV-positive disease are needed.
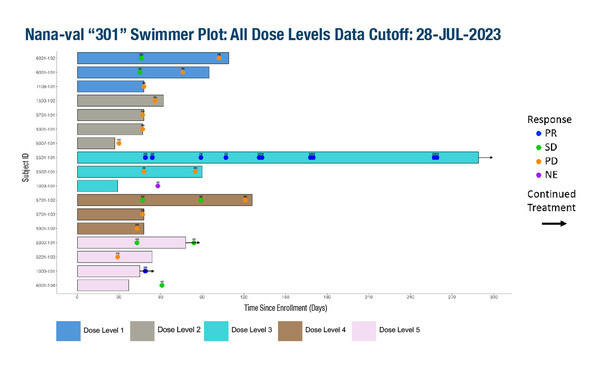
A phase Ib study involved 17 patients with no curative options who were enrolled across five dose-level cohorts: nanatinostat once daily at 20 mg (DL1), 30 mg (DL2) or 40 mg (DL3) for 4 days/week plus valganciclovir (900 mg once daily); or nanatinostat as a twice-daily dose of 10 mg (DL4) or as a 30-mg split daily dose (20 mg in the morning/10 mg in the afternoon) (DL5) for 4 days/week plus valganciclovir (900 mg twice daily for 21 days, then once daily).
Treatment-related adverse events (AEs) were mainly grade 1 or 2 and the most common were nausea and decreased appetite (n=7 each), increased creatinine (n=5) and fatigue (n=5). There were no treatment-related serious AEs or dose-limiting toxicities reported. Among patients evaluable for response, there was a case of partial response lasting over 9 months (at DL3).
The combination of nanatinostat and valganciclovir was tolerated at doses greater than the recommended phase II dose (RP2D) identified in the lymphoma study (nanatinostat 20 mg once daily, 4 days/week, plus valganciclovir 900 mg once daily). Further dose escalation with split daily dosing is planned. A phase Ib expansion cohort will enrol patients with other EBV-positive solid tumours and the phase II part of the trial will investigate the RP2D of the combination with or without pembrolizumab in EBV-positive recurrent/metastatic NPC (NCT05166577).
Abstract discussed:
Colevas AD, et al. A phase 1b/2 study of nanatinostat (Nstat) plus valganciclovir (VGCV) in EBV+ solid tumors and with pembrolizumab (PEM) in recurrent/metastatic nasopharyngeal carcinoma (R/M NPC). ESMO Asia Congress 2023, Abstract 354O
Proffered Paper Session: Head and Neck Cancer, 02.12.2023, h. 09:00 – 10:20 SGT, Hall 404