Results of a small phase II trial may open up a new option for some patients with leukoplakia, but toxicity of immunotherapy is high and questions remain about its utility in clinical practice
Results from a small single-arm study presented at ESMO Congress 2022 (Abstract 650O) showed potential in using nivolumab to reduce oral leukoplakia lesions in patients with a high risk of developing oral cancer. “This is the very first time an immunotherapy has shown some activity in this setting where treatment options are limited,” says Prof. Jean-Pascal Machiels, Head of the Department of Medical Oncology at Cliniques universitaires Saint-Luc, Brussels, Belgium. “Not all of these patients can be treated with surgery because even if a visible lesion is fully removed, some residual precancerous lesions may be left behind causing a risk for cancer recurrence.”
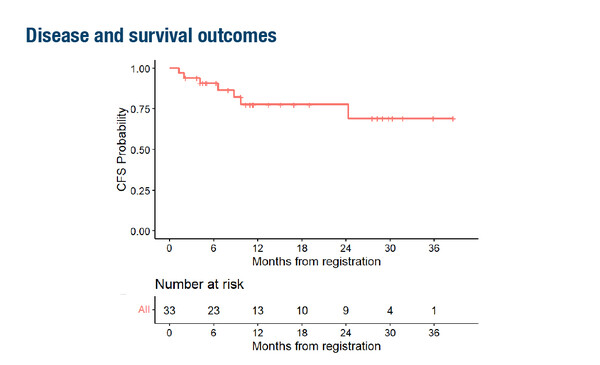
In the phase II study, nivolumab was given at 480 mg every 28 days for 4 doses, producing an overall response rate of 36.4%. At median follow-up of 14.7 months (range 4.1–39.9), median cancer-free survival (CFS) had not been reached; 2-year CFS was 77.7% (95% confidence interval [CI] 56.3–89.5) (Figure).
Patients in this study had oral proliferative leukoplakia that was multifocal, contiguous, or a single lesion ≥4 cm with any degree of epithelial dysplasia, and 27% had prior oral squamous cell carcinoma. The latter is important because, as Machiels explains, “Patients with a history of oral cancer are at high risk of relapse. However, not every patient with leukoplakia will develop cancer and, in this study, researchers selected a high-risk population.”
Toxicities in the study were significant; grade 3–4 adverse events including hyperglycaemia, colitis and hepatitis were reported in 21.2% of patients. Machiels is not overly concerned about this finding, remarking that: “We have learned how to manage these toxicities with steroids, so it is not such a major issue. Of course, it depends on patient selection – if they are at very, very high risk of progressing to cancer or their lesion is inoperable due to its extent, then the toxicity is acceptable in that subset of patients. If a patient has a small lesion, you will choose surgery in the first instance.”
Beyond balancing risks and benefits of treating oral leukoplakia, a key question is whether immunotherapy will delay or cure the disease. “It is too early to transfer into clinical practice,” concludes Machiels. “Also, what is not clear is how long you have to give immunotherapy for, and what would be the late recurrence rate. A randomised trial is now needed in a similar high-risk patient population to demonstrate that immunotherapy is really going to decrease the rate of oral cavity cancer. As this is a rare cancer, it will not be easy to do.”
Abstract presented:
Hanna GJ, et al. A phase 2 study of nivolumab for high-risk oral leukoplakia. ESMO Congress 2022, Abstract 650O
Proffered Paper Session – Head & neck cancer, 12.09.2022, h. 14:45 – 16:15, Évry Auditorium