Results from the BEATcc study strongly support the use of first-line combination therapy for R/M CC in all patients
Data presented at the ESMO Virtual Plenary in November confirm the importance of checkpoint and VEGF inhibition in metastatic, recurrent or persistent cervical cancer (R/M CC).
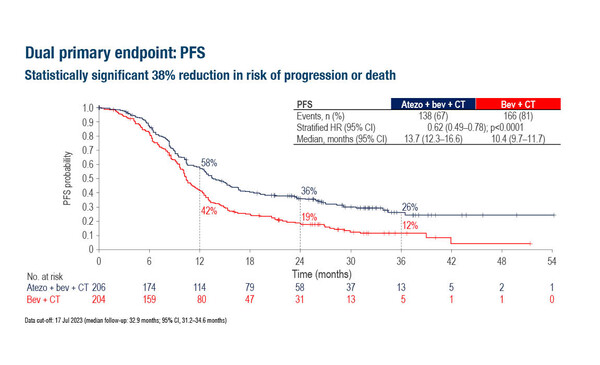
The BEATcc phase III trial reported the benefits of atezolizumab combined with the VEGF inhibitor bevacizumab and chemotherapy as a first-line treatment for R/M CC. Significant improvements were reported for patients randomised to atezolizumab (n=206) versus placebo (n=204) for both progression-free survival (PFS; stratified hazard ratio [HR] 0.62; 95% confidence interval [CI] 0.49–0.78; p<0.0001) and overall survival (OS; interim analysis HR 0.68; 95% CI 0.52–0.88; p=0.0046). For patients treated with atezolizumab, the median OS was 32.1 months and the objective response rate was 84%, compared with 22.8 months and 72%, respectively, for patients in the placebo arm. Grade ≥3 adverse events were reported for 79% of the atezolizumab arm and 75% of the placebo arm.
Commenting on these findings, Prof. Bradley J. Monk from University of Arizona College of Medicine, Creighton University School of Medicine, HonorHealth Research Institute, Phoenix, Arizona, USA, says, “The treatment of R/M CC has been transformed over the past 14 years, with a doubling of OS rates reported following the addition of bevacizumab in the GOG 240 trial (Lancet. 2017;390:1654–1663) and then pembrolizumab in the KEYNOTE-826 trial (J Clin Oncol. 2023;41(16_suppl):5500) compared with chemotherapy alone (J Clin Oncol. 2009;27:4649–4655). BEATcc is an important study as all the patients were treated with bevacizumab and the results confirm the data from the subgroup of patients in KEYNOTE-826 who received bevacizumab and pembrolizumab. These data strongly support the use of a checkpoint inhibitor combined with chemotherapy as a first-line treatment for R/M CC in all patients. Additionally, they tell us that all eligible patients should have bevacizumab included in their treatment regimen.”
Positive findings from the BEATcc trial adds further evidence to the key role played by checkpoint inhibitors in the treatment of cervical cancers, following the encouraging results from the KEYNOTE-A18 trial in high-risk, locally advanced disease recently presented at the ESMO Congress 2023.
Abstract discussed:
Oaknin A, et al. Primary results from BEATcc (ENGOT-Cx10/GEICO 68-C/JGOG1084/GOG-3030), a randomised phase III trial of first-line atezolizumab (atezo) combined with a platinum doublet and bevacizumab (bev) for metastatic (stage IVB), persistent or recurrent cervical cancer (R/M CC). (VP5-2023). ESMO Virtual Plenary, 3 November 2023.
On-demand recording of the full session and slides available to download for ESMO account holders