Final analyses of the PICCOLO trial confirm the efficacy of the antibody–drug conjugate in recurrent platinum-sensitive ovarian cancer
Positive primary findings from the phase II PICCOLO study, showing notable efficacy and acceptable tolerability of mirvetuximab soravtansine-gynx (MIRV) in heavily pretreated patients with FRα-positive platinum-sensitive ovarian cancer (PSOC) (Ann Oncol. 2025;36:321–330), were confirmed with a final analysis presented at the ESMO Gynaecological Cancers Congress 2025 (Vienna, 19–21 June) (Abstract 76MO).
MIRV is an FRα-targeting antibody–drug conjugate (ADC) that received full US FDA approval in March 2024 for patients with FRα-positive platinum-resistant epithelial ovarian cancer (PROC) with 1–3 prior lines of systemic therapy, supported by results from the phase III MIRASOL trial (N Engl J Med 2023;389:2162–2174).
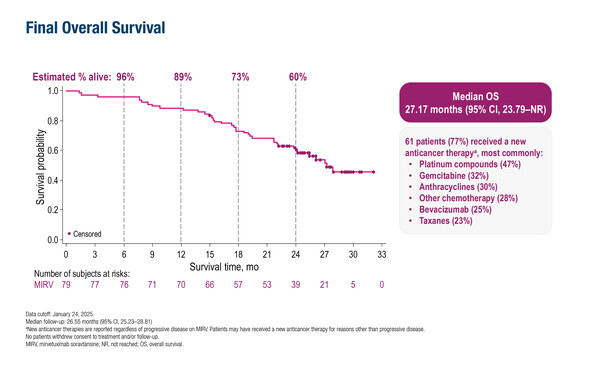
Final data from the PICCOLO trial showed that at a median follow-up of 26.6 months, median progression-free survival (PFS) was 6.9 months (95% confidence interval [CI] 5.9–9.6) and median overall survival (OS) was 27.2 months (95% CI 23.8–not reached). In total, 79 patients received MIRV as third-line or greater treatment in patients with FRα-positive (≥75% of cells with ≥2+ staining intensity) PSOC.
The objective response rate (ORR) was 51.9% and the median duration of response (DOR) was 8.3 months. MIRV demonstrated a higher ORR among PARP inhibitor-naïve patients (75.0%) than in those previously exposed to a PARP inhibitor (46.9%). The most common treatment-emergent adverse events (TEAEs) were gastrointestinal, neurosensory and resolvable ocular events, in line with the primary analysis. TEAEs led to discontinuation in 13 participants (16%) and death in 2 participants (3%).
Prof. Jean-Emmanuel Kurtz from the Institut de Cancérologie Strasbourg Europe (ICANS) and Hôpitaux Universitaires de Strasbourg, France, thinks the PICCOLO findings “are promising” given that the likelihood of responding to a new platinum-based combination in heavily pre-treated patients with PCOS is around one-fifth to one-quarter. “However, the study does not tell us whether MIRV is able to improve the efficacy of subsequent platinum treatments and time to subsequent therapy by extending the platinum-free interval. This would be interesting to explore as it may help us to achieve better responses in the setting of platinum-sensitive disease,” he notes.
Also reported at the Congress, additional data from a PICCOLO subgroup analysis highlighted that MIRV demonstrated efficacy in patients with or without homologous recombination deficiency (HRD) (Abstract 72MO). The HRD-positive subgroup (n=19) had a numerically higher ORR (57.9% versus 42.1%) but a shorter median DOR (5.8 months versus 8.3 months), with a similar median PFS (6.2 months versus 6.9 months), compared with the HRD-negative subgroup (n=19).
Kurtz thinks these data may suggest that MIRV is not restricted to a specific HRD subgroup, although they do not provide any further information about a possible difference in efficacy between patients with and without prior PARP inhibitor exposure. “In the study, almost all participants had received a PARP inhibitor – 84% of HRD-positive and 79% of HRD-negative patients – and there are no data on the interval between the end of PARP inhibitor treatment and recurrence. This is important as it likely influences response to subsequent treatment in a similar way to platinum-based chemotherapy,” he observes.
Programme details
Oaknin A, et al. Homologous recombination deficiency (HRD) and genomic subgroup analysis in patients (pts) with platinum-sensitive ovarian cancer (PSOC) receiving mirvetuximab soravtansine-gynx (MIRV) in the PICCOLO clinical trial. ESMO Gynaecological Cancers Congress 2025, Abstract 72MO
Mini Oral Session, 20.06.2025, h. 10:45 – 12:00, Hall D
Secord A, et al. Final analysis of the single-arm phase 2 PICCOLO trial of mirvetuximab soravtansine-gynx (MIRV) in folate receptor alpha (FRα)-positive, third-line and later (3L+), recurrent platinum-sensitive ovarian cancer (PSOC). ESMO Gynaecological Cancers Congress 2025, Abstract 76MO
Mini Oral Session, 20.06.2025, h. 10:45 – 12:00, Hall D