The ROSELLA trial highlights the efficacy and tolerability of the combination even in patients with a poorer prognosis
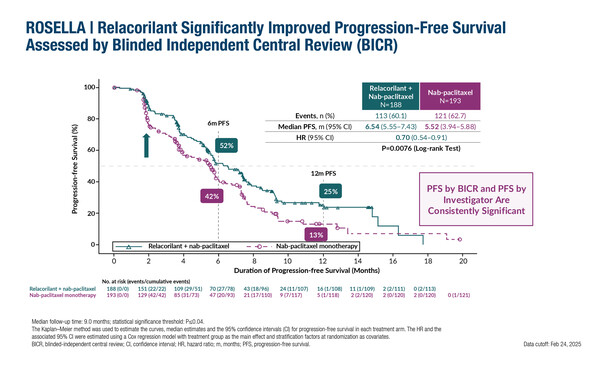
The selective glucocorticoid receptor antagonist, relacorilant, plus nab-paclitaxel significantly improved progression-free survival (PFS) assessed by blinded independent central review compared with nab-paclitaxel monotherapy (hazard ratio [HR] 0.70; 95% confidence interval [CI] 0.54–0.91; log-rank p=0.0076), according to results presented at the ESMO Gynaecological Cancers Congress 2025 (Vienna, 19–21 June) (Abstract 70O). The phase III ROSELLA trial included 381 patients with ovarian cancer who had progressed less than 6 months after their last dose of platinum therapy.
Based on an interim analysis, there was a clinically meaningful improvement in overall survival (OS) with relacorilant plus nab-paclitaxel versus nab-paclitaxel alone (HR 0.69; 95% CI 0.52–0.92; nominal p=0.0121). In an exploratory analysis, the addition of relacorilant to nab-paclitaxel showed a consistent benefit across prognostic subgroups in those with a particularly poor prognosis, including in 112 patients with a primary platinum-free interval of ≤6 months (PFS: HR 0.50; 95% CI 0.30–0.84; interim OS: HR 0.52; 95% CI 0.31–0.89).
Commenting on the efficacy data, Dr Robert L. Coleman from The Woodlands, Houston, TX, USA, says, “The proportion of patients without progression at 12 months in the relacorilant plus nab-paclitaxel group was almost double that in the nab-paclitaxel group – 25% versus 13% – which is a very positive signal for activity. And the OS data, which are around 50% mature, appear promising.” He highlights the strong rationale for the combination: “Activation of the glucocorticoid pathway has been shown to reduce the efficacy of chemotherapy by inhibiting drug-induced apoptosis. Preclinical experiments and limited clinical data suggested that blocking the glucocorticoid receptor (GR) with agents such as relacorilant could mitigate this effect, potentiating cytotoxicity in breast and ovarian cancer models (Clin Cancer Res. 2009;15:3196–3204). Weekly nab-paclitaxel was developed to avoid some of the common taxane toxicities, including hypersensitivity reactions and gastrointestinal symptoms – adverse effects commonly treated with steroid pre-medication. This enabled the hypothesis that combining these two factors, GR regulation by relacorilant and a steroid-free taxane (nab-paclitaxel), could lead to better efficacy in patients with ovarian cancer.”
Grade ≥3 treatment-emergent adverse events were reported in 74.5% of patients with relacorilant plus nab-paclitaxel and 59.5% with nab-paclitaxel alone. Coleman notes that when adjusting for the longer duration of exposure with combination therapy, the safety profile appeared broadly similar between the groups “suggesting minimal adverse impact of relacorilant on tolerability.”
Acknowledging the hazards of cross-trial comparisons, Coleman summarises that the findings of the ROSELLA trial compare favourably with those seen in the MIRASOL trial, which assessed the antibody–drug conjugate mirvetuximab soravtansine-gynx in a biomarker-selected population with FR)α-positive platinum-resistant ovarian cancer (N Engl J Med. 2023;389:2162–2174) – the first agent to demonstrate an OS improvement in this difficult-to-treat patient population. The ongoing KEYNOTE-B96 trial of pembrolizumab versus placebo in combination with paclitaxel, with or without bevacizumab, is assessing another novel strategy in platinum-resistant recurrent ovarian cancer, with results expected later this year (NCT05116189). “The results of the MIRASOL, ROSELLA and KEYNOTE-B96 trials can then be interpreted in context with each other to identify the most effective regimens overall, sequencing strategies and options for different subpopulations,” he concludes.
Programme details
Lorusso D, et al. Phase III results of relacorilant + nab-paclitaxel vs nab-paclitaxel in platinum-resistant ovarian cancer (PROC) (ROSELLA, GOG-3073, ENGOT-ov72): Secondary endpoints. ESMO Gynaecological Cancers Congress 2025, Abstract 70O
Proffered Paper Session, 19.06.2025, h. 14:00 – 15:30, Hall D