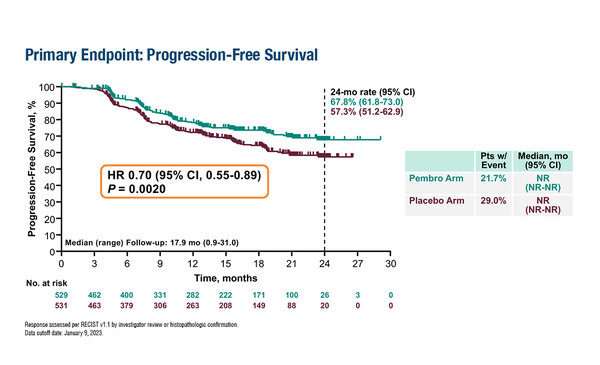
In the KEYNOTE-A18 study, the combination of pembrolizumab and concurrent chemoradiotherapy significantly improved progression-free survival
Data presented at the ESMO Congress 2023 (Madrid, 20–24 October) reveal that checkpoint inhibitors can improve outcomes in high-risk, locally advanced cervical cancer for the first time (LBA38). The encouraging results were reported for the combination of pembrolizumab and concurrent chemoradiotherapy (CCRT) in the KEYNOTE-A18 trial, showing significant improvements in progression-free survival (PFS) – the study’s co-primary endpoint – for patients who received pembrolizumab and CCRT versus placebo and CCRT (HR 0.70; 95% CI 0.55–0.89; p=0.0020).
OS data are currently immature, but a favourable trend was reported for the pembrolizumab arm (HR 0.73; 95% CI 0.49–1.07). In addition, the combination of pembrolizumab and CCRT was shown to have a manageable safety profile. “This is very exciting as it is the first positive study we have had in locally advanced cervical cancer since 1999, suggesting that the use of checkpoint inhibitors in locally advanced cervical cancer is the next frontier for this disease,” says Prof. Bradley J. Monk from University of Arizona College of Medicine, Creighton University School of Medicine, HonorHealth Research Institute, Phoenix, Arizona, USA, commenting on the data.
The findings presented in Madrid are in contrast to results from the phase III CALLA trial of the PD-L1 inhibitor durvalumab in the same setting published last year, which were disappointing (Int J Gynecologic Cancer. 2022;32(Suppl 3):A2–A3). “It is unclear why the KEYNOTE-A18 study was positive when CALLA was negative, but it could be due to differences in sample size or because the patients in KEYNOTE-A18 were a higher-risk population,” continues Monk. “Minor differences in the mechanism of action between PD-1 and PD-L1 inhibitors may also account for differences in efficacy.” In the same setting, results of the GCIG INTERLACE trial investigating induction chemotherapy before CCRT, which will be presented in a Presidential Symposium at the ESMO Congress 2023, are eagerly awaited.
In conclusion, Monk highlights, “It is now clear that checkpoint inhibitors play a critical role in the treatment of cervical cancer. They are effective as both second-line and first-line treatments for metastatic, recurrent or persistent cervical cancer and we see that they work with chemotherapy and radiotherapy in locally advanced cancers.” Looking to the future, he adds, “For patients who progress on standard of care treatments, we urgently need novel therapies. Antibody–drug conjugates targeted against molecules expressed on cervical cancers such as tissue factor or HER2 are a very promising strategy and offer hope to patients with cervical cancer and disease progression.”
Abstract discussed:
Lorusso D, et al. Pembrolizumab plus chemoradiotherapy for high-risk locally advanced cervical cancer: A randomized double-blind, phase 3 ENGOT-cx11/GOG-3047/KEYNOTE-A18 study. ESMO Congress 2023, LBA38
Proffered Paper Session 1 – Gynaecological cancers, 20.10.2023, h. 16.00 – 17.30, Sevilla Auditorium – Hall 9