Despite being active in this disease setting, the antibody-drug conjugate was associated to high rates of neutropenic complications
Final results of the phase III TROPiCS-04 study showed that sacituzumab govitecan was active in pre-treated patients with advanced urothelial carcinoma (UC), but did not demonstrate to significantly prolong overall survival (OS) or progression-free survival (PFS) compared to platinum-based chemotherapy (Ann. Oncol. 2024 Dec;35(4):S1505-S1507). The OS and safety analysis were presented at the ESMO Asia Congress 2024 earlier this month (Singapore, 6-8 December).
Sacituzumab govitecan is a Trop-2-directed antibody-drug conjugate which has previously shown a promising antitumour activity and a manageable toxicity profile in pretreated patients with advanced UC, either as a single agent (J Clin Oncol. 2021 Aug 1;39(22):2474-2485) or in combination with pembrolizumab (J Clin Oncol. 2024 Apr 20;42(12):1415-1425). In the global, open-label TROPiCS-04 trial, 711 patients with metastatic or locally advanced UC, who had progressed on or after platinum-based chemotherapy and anti-PD-(L)1 therapy, were randomised to either receive sacituzumab govitecan (10 mg/kg intravenously, on days 1 and 8, every 21 days) or platinum-based chemotherapy (physician’s choice, intravenous paclitaxel 175 mg/m2, docetaxel 75 mg/m2, or vinflunine 320 mg/m2, on day 1 every 21 days).
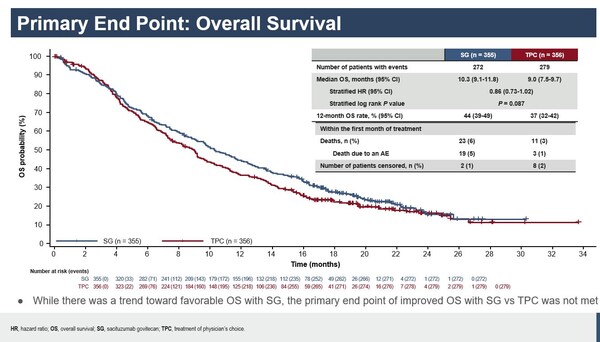
After a median follow-up of 9.2 months, while a trend toward favorable OS with sacituzumab govitecan was observed, the primary endpoint of improved OS with the antibody-drug conjugate vs chemotherapy was not met (OS of 10.3 vs 9.0 months respectively; HR, 0.86; 95% CI,0.73-1.02; 2-sided P = 0.087). Hazard ratios of OS consistently favoured sacituzumab govitecan vs chemotherapy in most prespecified subgroups. No significant PFS improvements were also reported in the antibody-drug conjugate group.
Safety data were consistent with the known toxicity profile of sacituzumab govitecan across tumour types. However, grade 5 treatment-emergent adverse events (TEAEs) were observed in 7% of patients treated with the antibody-drug conjugate while occurring in 2% of patients in the chemotherapy group. Sacituzumab govitecan was associated with increased rates of infections in the setting of neutropenia, mainly occurring within the first month of treatment. Patients who experienced fatal infections with neutropenia had a higher burden of risk factors for medical complications compared with the overall treatment group (age ≥ 65 years, prior cystectomy, prior major urinary tract procedure, prior radiotherapy, at least 3 prior anticancer regimens). According to the study researchers, a low usage of primary prophylactic granulocyte colony stimulating factor (G-CSF), which was not required per study protocol, may have impacted the safety data.
Abstract details
Grivas P, et al. TROPiCS-04: A randomized phase III study of sacituzumab govitecan (SG) vs chemotherapy (CT) in pretreated advanced urothelial carcinoma (aUC).
ESMO Asia Congress 2024, LBA9, https://doi.org/10.1016/j.annonc.2024.10.830