In an interview, Prof. Julien Taieb comments on the major advances from colorectal cancer research which are driving the development of molecular-based therapies
The high heterogeneity of colorectal cancer (CRC) revealed by molecular testing has opened-up new opportunities of care for difficult-to-treat molecular subtypes, with an impressive development of biomarker-driven targeted therapies that are rapidly shaping how patients are treated at any stage of disease trajectory.
According to Prof. Julien Taieb, Head of the Gastroenterology and Gastrointestinal Oncology Department at the Georges Pompidou European Hospital, Université Paris-Cité, France, and co-author of the 2023 update of the ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up of metastatic colorectal cancer, precision oncology for gastrointestinal cancers is keeping up with advances made in other tumours, but further efforts are now needed to overcome some obstacles in how research is conducted.
How has an increasing knowledge of the colorectal cancer molecular landscape impacted on treatment and prognosis?
In the last years, we have seen remarkable progress in the development of molecular-based therapies leading to very positive outcomes in some genetic subtypes.
For instance, the paradigm of care has significantly changed for colorectal cancers that show deficiencies in the mismatch repair machinery and high microsatellite instability (dMMR/MSI-H). They represent approximately 10 to 15% of non-metastatic and 5% of metastatic colorectal cancers. Being hyper-mutated, they generate a lot of antigens that trigger the immune system, so restoring inefficient immune reactions against the tumour has proved to be a feasible as well as effective strategy.
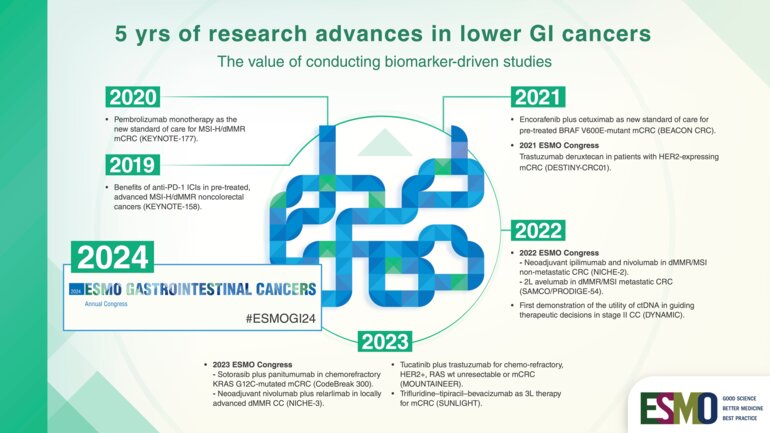
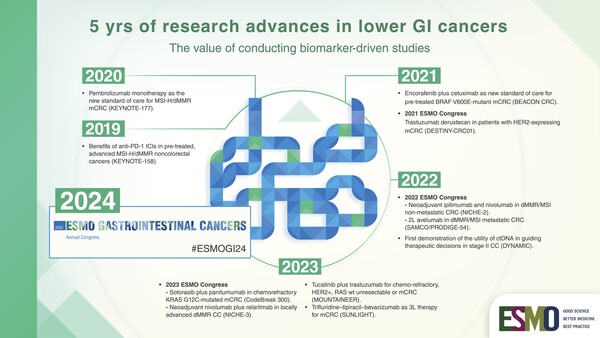
Following some initial studies providing a proof of concept of the role of immunotherapy in this setting (N Engl J Med 2015;372:2509-2520; Science 2017;357:409-413), the KEYNOTE-177 study provided conclusive evidence showing that first-line pembrolizumab is superior to chemotherapy plus a targeted agent in patients with MSI-H/dMMR metastatic colorectal cancer (N Engl J Med 2020; 383:2207-2218). Study results led pembrolizumab to be approved as a new standard of care in the first-line setting and to its reimbursement in many countries.
Most recently, a second randomised trial, the SAMCO / PRODIGE-54, also showed an improvement in progression-free survival and other endpoints with the use of the PDL-1 inhibitor avelumab in second-line for dMMR/MSI metastatic CRC patients (JAMA Oncol. 2023 Aug 3;e232761).
Despite many dMMR/MSI-H CRCs show primary or acquired resistance to immunotherapy, today almost one third are long-responders – at more than 2 years –, but further research is needed to assess how long they will continue to respond to immunotherapy and how to overcome primary and secondary resistance to Immune Checkpoints inhibitor(s) (ICI).
Now research is rapidly moving to neoadjuvant setting with ICI based on encouraging results from some recent studies such as the NICHE-2 trial presented at ESMO Congress 2022 which showed that delivering a short period of immunotherapy to patients with non-metastatic dMMR colon cancer before resectable cancer surgery is not only feasible, but also has outstanding efficacy. Neoadjuvant immunotherapy may completely transform the landscape of treatment from the early to late stages of colon and rectal cancer in the future, so conclusive data from some ongoing studies like IMOTHEP are eagerly awaited (NCT04795661).
What targets do still represent a major challenge in the treatment of metastatic colorectal cancer?
Unlike MSI, targeting other mutations has recently come with mixed findings. Today, we have some targeted agents that are working on BRAF-mutant colorectal cancer patients - a population with a poor prognosis -, but they are not effective when used alone. Unlike in mutated-melanoma, CRC carrying BRAF V600E mutation seem to react to BRAF inhibitors only when the upregulation of EGFR is blocked with an anti-EGFR. This was confirmed for the first time by the results from the phase III BEACON trial showing that a combination of three targeted agents—encorafenib, cetuximab and binimetinib—leads to improved outcomes for patients with metastatic colorectal cancer positive for the BRAF V600E mutation who have failed first-line treatment. Currently, recommendations are to use the doublet encorafenib/cetuximab targeted therapy as standard therapy in second or third line (Metastatic Colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up), and we are eagerly awaiting to see the results of the BREAKWATER phase III trial which is investigating the combination therapy plus chemotherapy in first-line (NCT04607421).
Another difficult-to-treat patient population is RAS-mutant CRC. In our multidisciplinary meetings having a patient MSS and RAS mutant is still a bit daunting because anti-EGFR, anti-BRAF and immunotherapy have proven not to be effective in these patients.
However, treatment targeting the KRAS pathway is now improving with KRASG12C inhibitors which are already quite well developed. Early-phase trial results were reported for sotorasib, adagrasib and divarasib that have been tested in cohorts of colorectal cancer patients, showing improved results when combined with an anti-EGFR like in BRAF-mutant tumours (Annals of Oncology (2022) 33 (suppl_7): S136-S196). Although the activity in CRC in terms of radiological responses seems somehow limited, results are encouraging with a progression-free survival around 6 months and some response even in pre-treated patients, which is not so easy to obtain in CRC. Also, some agents targeting G12D and G12V mutations are under development. This represent an important step forward because G12C mutation accounts for only 3% of colorectal cancer, while G12V and G12D mutations are more frequent – 10-12% incidence.
There is still a lot of research to be done, and in the forthcoming years we will see if findings from randomised trials (CODEBREAK 300 study results awaited for ESMO 2023 testing sotorasib and panitumumab vs SOC in pre-treated KRAS G12C mutant mCRC patients) will confirm the proof of concept we currently have.
What advances do you expect from research in colorectal cancer in the next future?
Biomarker testing will continue to impact on how colorectal cancers are managed especially in the metastatic setting where treatment will be soon very different from one molecular subgroup to the other. Also, the efficacy of the use of targeted agents in the (neo)adjuvant setting will be assessed and we will see whether better outcomes can be achieved by using them earlier in the disease journey.
Another major advancement will be made by circulating tumour DNA to decide adjuvant therapies and when we may avoid chemotherapy for some patients. This is a very exciting field for GI researchers and many trials are ongoing all over Europe and globally, with very impressive results already coming from Japan and Australia (Nat Med 29, 127–134 (2023); N Engl J Med 2022; 386:2261-2272)
What are still the main obstacles to precision oncology for colorectal cancer?
Next-generation sequencing (NGS) has enabled oncologists to identify tumour biomarkers reliably and testing is now easily performed in many clinics as a routine task. Compared to first-generation approaches, NGS is not so expensive, so costs can be covered in many countries. However, there are some organisational and regulatory issues that limit molecular analysis. One such is a trend toward centralisation of the molecular profiling in some multicentric clinical trials, instead of having it performed at a single centre level, which often prolongs the waiting time for starting treatments based on the NGS results of patients. In Europe, another issue is the recent laboratory quality control and accreditation procedures set up by the EU, which set difficult-to-reach standards for academic laboratories, thus resulting in very time-consuming routine tasks and impairing our continental academic research performances. All these issues have nothing to do with making quality cancer care available and accessible to our patients, but do slow down the progress of research which is also crucial in oncology.
ESMO Gastrointestinal Cancers Congress 2024
In recent years we have seen remarkable progress in the treatment options available for patients with GI cancers and ESMO is committed to ensuring that there is a clear roadmap to help facilitate the implementation of new practice changing discoveries in the clinic for the benefit of all patients with GI cancers and whose wellbeing is our primary concern. The ESMO Gastrointestinal Cancers Congress 2024 will take place from 26 to 29 June in Munich, Germany.