Although not immediately practice-changing, impressive results from the NICHE-2 trial may open a way to a ‘watch and wait’ approach for some patients
Encouraging results from the NICHE-2 trial, as reported at ESMO Congress 2022 set the basis for larger trials to confirm the benefits of immune checkpoint inhibition in patients with mismatch repair-deficient (dMMR) colon cancer (LBA7).
The effectiveness of neoadjuvant immunotherapy in patients with dMMR colon cancer was shown for the first time in the exploratory NICHE trial in which pathological responses was achieved in 100% of dMMR tumours (Nat Med. 2020;26:566–576), indicating the feasibility of this approach and paving the way for a larger trial.
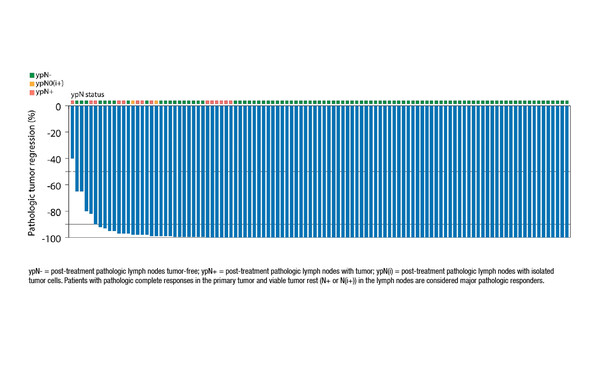
In the NICHE-2 trial, 112 patients with non-metastatic dMMR colon cancer received one dose of ipilimumab (1 mg/kg) and two doses of nivolumab (3 mg/kg) ≤6 weeks prior to surgery. Co-primary endpoints were safety and 3-year disease-free survival (DFS), with secondary endpoints including major pathological response (MPR) and pathological complete response (pCR). DFS data are yet to be reported, but the primary endpoint of safety was met, with 4% of patients experiencing grade 3–4 immune-related adverse events and 2 patients experiencing a delay in surgery ≥2 weeks. With a median 5.4 weeks from first dose to surgery, pathological response was observed in 99% of patients, with MPR and pCR achieved in 95% and 67% of patients, respectively. No patients had disease recurrence after a median follow-up of 13.1 months.
“These data have the potential to be paradigm changing,” says Dr Jenny Seligmann from the University of Leeds, UK. “NICHE-2 further emphasises that for patients with dMMR early colon cancer, future treatment schedules will almost certainly be with immunotherapy rather than chemotherapy. It opens the possibility that a non-operative surveillance approach may be possible for some patients with dMMR early colon cancer.”
Whether these data support the use of neoadjuvant immunotherapy as standard of care in early colon cancer, Seligmann says that caution is warranted. “The NICHE-2 study shows that delivering a short period of immunotherapy before resectable cancer surgery is feasible, has spectacular efficacy – with pathological responses unlike anything we have seen in colon cancer over the last 10 years – and acceptable toxicity,” she states. “However, before clinical practice can change, we need clarification on several aspects. Firstly, the trial was conducted in a single centre where there is great expertise in patient selection and treatment delivery and committed multidisciplinary teams, so confirmation of these results in other settings is urgently needed. Also, as we await data for the co-primary endpoint of 3-year DFS, longer term follow-up data are needed to confirm the initial results.”
The incidence of dMMR is approximately 15% in patients with colorectal cancer (Cancers (Basel). 2021;13:300), therefore, patient selection for clinical trials such as NICHE-2 is key. Zorana Maravic from Digestive Cancers Europe (DiCE), Brussels, says, “Clinical studies are an important treatment option for patients with cancer, and for some, they are the only option. From the perspective of DiCE and the patients we work with, there is considerable concern that there are not enough study options offered to patients, particularly in some European countries.” Conducting research in subpopulations of patients identified by molecular patterns can be difficult, resulting in delays to promising findings being translated into approved treatments. Maravic continues, “There is a substantial discrepancy between the information doctors believe they are giving to patients about the molecular characterisation of their tumour and what patients know and understand of it. Patients are often unaware that tumour characterisation has been performed and the impact this might have on future treatment decisions. Consequently, it is difficult for them to know if they are suitable candidates for some clinical trials.”
Describing other obstacles and challenges to patients’ participation in clinical research, Maravic adds, “Often the available clinical trial databases do not contain complete information on suitable trials, or this information may not be up to date. Another problem is the unavailability of clinical trials in some countries, where patients may need alternative treatment options that clinical research could offer. Lastly, participation in clinical research, which could be an important option in the continuum of treatment and care, is something that patients feel is not sufficiently discussed with them by their treating physicians and more information should be offered.”
In terms of future research, Seligmann notes, “It may not be acceptable to conduct a trial where dMMR early colon cancer patients are randomised between neoadjuvant immunotherapy or chemotherapy, especially as the FOxTROT trial clearly showed a lack of benefit with neoadjuvant chemotherapy in these patients. However, both ipilimumab and nivolumab may not be needed for all patients, and instead this may be a more sensible approach. Another major consideration for use of neoadjuvant immunotherapy relates to patient selection. In the adjuvant setting, patient selection is made according to post-operative pathology, whereas in the neoadjuvant setting, decisions are based on CT assessment of the tumour as well as the biopsy, and we know radiological assessment of lymph nodes is particularly difficult in dMMR cancers. This is an area that needs development while we wait for the final results of the NICHE-2 trial. However, a huge advantage of the neoadjuvant setting over the adjuvant or metastatic setting is the potential for translational research to identify predictive biomarkers in pre- and post-treatment samples, to identify those who require combination immunotherapy.”
Abstract presented:
Chalabi M, et al. Neoadjuvant immune checkpoint inhibition in locally advanced MMR-deficient colon cancer: the NICHE-2 study. ESMO Congress 2022, LBA7
Presidential Symposium II, 11.09.2022, h. 16:30 – 18:15, Paris Auditorium