A triple combination investigated in a phase I trial adds to the growing therapeutic options directed at this emerging target
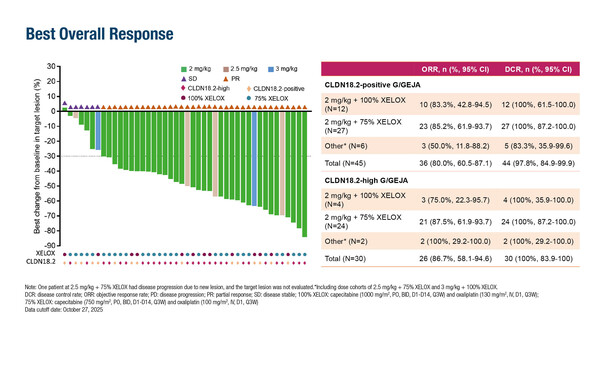
JS107, a claudin 18.2 (CLDN18.2)-targeting antibody–drug conjugate (ADC), in combination with toripalimab and XELOX chemotherapy, achieved an impressive response rate in the first-line treatment of advanced CLDN18.2-high gastric or gastro-oesophageal junction adenocarcinoma (G/GEJA) in a Chinese phase I trial presented at the ESMO Asia Congress 2025 (Singapore, 5–7 December) (LBA5). Amongst 45 patients with CLDN18.2-positive G/GEJA (defined by staining intensity ≥1+ in ≥1% of tumour cells), 30 patients had high CLDN18.2 expression (staining intensity ≥2+ in ≥40% of tumour cells) and these patients achieved an objective response rate of 86.7%, with a 100% disease control rate. Median progression-free survival (PFS) was 11.14 months in patients with high CLDN18.2 expression after a median follow-up of 7.1 months. Median overall survival data are immature.
In recent years, the development of CLDN18.2-targeted therapies has expanded rapidly, including monoclonal antibodies, ADCs, chimeric antigen receptor (CAR) T-cell therapy and mRNA-based approaches. “The most well-known CLDN18.2-targeting agent is the antibody, zolbetuximab, which is an approved first-line addition to chemotherapy based on positive data from the SPOTLIGHT (Lancet. 2023;401:1655–1668) and GLOW (Nat Med. 2023;29:2133–2141) phase III trials in CLDN18.2-positive patients,” says Dr Victor Moreno from START Madrid-FJD, Hospital Fundación Jiménez Díaz, Madrid, Spain. “Here, the researchers have made a further step forward by conjugating an anti-CLDN18.2 antibody to monomethyl auristatin E, resulting in impressive early efficacy. The planned phase III trial will provide further confirmation and also corroborate if the safety profile of the triple combination is indeed manageable, which appeared to be the case in phase I.”
In the presented trial, a dose of JS107 2 mg/kg, in combination with 100% and 75% XELOX plus toripalimab, was chosen for the dose-expansion cohort. One patient from the dose-escalation cohort who received JS107 3 mg/kg plus 100% XELOX experienced dose-limiting toxicities (grade 3 febrile neutropenia and grade 3 diarrhoea). Grade ≥3 treatment-related adverse events occurred in 55.6% of patients with JS107 2 mg/kg, 75% XELOX plus toripalimab and in 58.3% of patients with JS107 2 mg/kg, 100% XELOX plus toripalimab. These were most commonly decreased neutrophil and platelet counts.
“This is a very active area,” Moreno explains, “particularly because of the high gastric expression of CLDN18.2 and its changing distribution during malignant transformation (Clin Cancer Res. 2008;14:7624–7634).” He highlights the results of a phase II study with the CLDN18.2-targeted CAR T-cell therapy, satricabtagene autoleucel (CT041) – the first positive, randomised controlled trial of CAR T-cell therapy in solid tumours globally – where a significant improvement in PFS was observed in patients with previously treated G/GEJA (Lancet. 2025;405:2049–2060). He concludes, “JS107 was tested with immunotherapy and chemotherapy in previously untreated patients, and I think we will see more of this early intensive approach in the future.”
Programme details:
Xu R-H, et al. Updated results from a phase I study of JS107, a claudin 18.2 (CLDN18.2)-targeting antibody-drug conjugate (ADC), in combination with toripalimab and chemotherapy as the first-line treatment of advanced gastric or gastroesophageal junction adenocarcinoma (G/GEJA). ESMO Asia Congress 2025 - LBA5