Encouraging data were reported for SHR-1701 added to first-line CAPOX in a post-hoc analysis of patients with high-risk features from a Chinese study
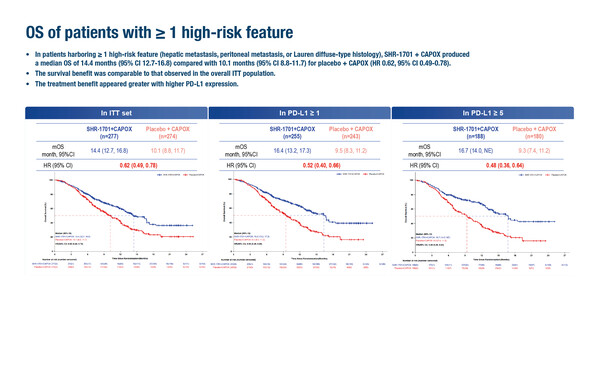
Post-hoc analysis of a phase III study showed favourable results with the PD-L1/TGF-β-targeting bifunctional fusion protein SHR-1701 in addition to first-line CAPOX in patients with gastric/gastro-oesophageal junction cancer (GC/GEJC) and liver metastases, diffuse-type histology or peritoneal metastases (Abstract 243MO). A presentation at the ESMO Immuno-Oncology Congress 2025 (London, 10–12 December) reported that overall survival (OS) was 14.4 months with CAPOX plus SHR-1701 versus 10.1 months with CAPOX plus placebo (hazard ratio [HR] 0.62; 95% confidence interval [CI] 0.49–0.78) among 551 patients with previously untreated, unresectable locally advanced or metastatic HER2-negative GC/GEJC and at least one high-risk feature. The HR in this post-hoc analysis – which included 75.4% of the intention-to-treat population – was similar to that reported for all 731 patients included in the previously reported primary analysis (HR 0.66) (Ann Oncol. 2024;35(Suppl 2);S1250). The analysis involved relatively small numbers of patients, especially for diffuse-histology disease, and was conducted only in a Chinese population.
“Looking at these results in isolation, it is encouraging to see that SHR-1701 remains active in a group of patients with high-risk disease features, particularly those with diffuse-type histology, who have a particularly poor prognosis and a high unmet need for effective treatments,” observes Prof. Francesco Sclafani from Institut Jules Bordet – HUB, Belgium. Despite these encouraging findings, he highlights that questions remain about the place of this novel combination in therapy. “We need to ask what degree of additional efficacy is being achieved with SHR-1701’s co-targeting of TGFβ?” he says, noting that the control arm included chemotherapy alone, while the combination of chemotherapy with a PD-1 checkpoint inhibitor, including pembrolizumab (Lancet Oncol. 2023;24:1181–1195), nivolumab (Lancet. 2021;398:27–40) and tislelizumab (BMJ. 2024:385:e078876) is a standard of care in this setting, with levels of benefit that appear largely similar to those seen with SHR-1701.
In the post-hoc subgroup analysis, benefits according to individual high risk factors were similar, showing a median OS with SHR-1701 compared with placebo of 16.8 months versus 10.3 months in 351 patients with liver metastases (HR 0.46; 95% CI 0.34–0.63), 12.6 months versus 8.4 months in 247 patients with peritoneal metastases (HR 0.68; 95% CI 0.49–0.95) and 16.4 months versus 9.5 months in 80 patients with diffuse-type histology (HR 0.50; 95% CI 0.26–0.95).
“Bearing in mind the small numbers, results are especially provocative for patients with diffuse-type tumours, which are known to be characterised by activation of the TGF-β pathway and an immune-suppressive stroma. Independent larger series are needed to confirm these hypothesis-generating findings. Also, it would be interesting to assess SHR-1701 against novel agents targeting claudin 18.2, a biomarker that is enriched in these tumours,’’ he concludes.
Programme details:
Peng Z, et al. SHR-1701 plus CAPOX for advanced gastric or gastroesophageal junction adenocarcinoma with high-risk features: Results from a phase III study. ESMO Immuno-Oncology Congress 2025 - Abstract 243MO