Benefits were seen across most predefined subgroups investigated in the practice-changing POD1UM-303/InterAACT-2 study
Mature survival data from the phase III POD1UM-303/InterAACT-2 trial confirm the role of first-line PD-1 inhibition plus chemotherapy as a standard of care for advanced squamous cell anal cancer (SCAC). SCAC represents just 3% of all gastrointestinal malignancies, but the prognosis for those with metastatic disease is poor, with a 5-year overall survival (OS) rate of only around 30% (Ann Oncol. 2021;32:1087–1100).
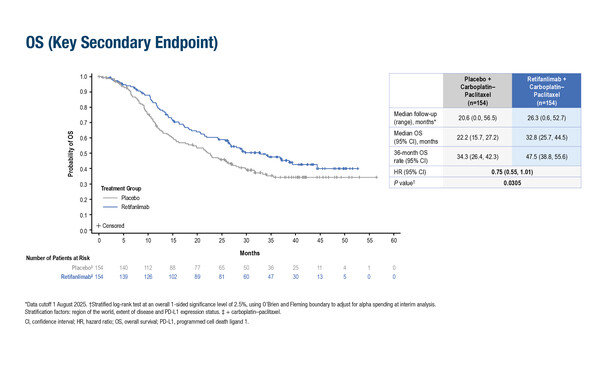
According to findings presented at the ESMO Immuno-Oncology Congress 2025 (London, 10–12 December), the addition of retifanlimab to carboplatin–paclitaxel (CP) led to a 25% reduction in the risk of death compared with placebo plus CP (hazard ratio [HR] 0.75; 95% confidence interval 0.55–1.01; p=0.0305) among 308 patients with locally recurrent or metastatic SCAC (Abstract 123MO). Median OS was 32.8 months with retifanlimab versus 22.2 months with placebo at a median follow up of 26.3 months and 20.6 months, respectively. Patients receiving placebo plus CP were allowed to crossover to retifanlimab monotherapy on disease progression. Adjusting for this crossover, the difference in OS between the arms increased to around 15 months in favour of retifanlimab (32.8 months versus around 17.0 months with placebo).
“It is hard to overestimate the importance of this OS analysis,” says Dr Erika Martinelli from the University of Campania Luigi Vanvitelli, Naples, Italy, commenting on the results of this first phase III trial with a checkpoint inhibitor combined with chemotherapy in SCAC. “Successfully conducting this practice-changing study is, in itself, an achievement in this rare disease. Following on from the primary analysis that showed a significant improvement in progression-free survival (Lancet. 2025;405:2144–2152), new findings from POD1UM-303 consolidate the benefit of adding retifanlimab to chemotherapy in the first-line treatment of advanced SCAC.”
OS benefits were seen across most predefined subgroups investigated. Although HRs were not as favourable for PD-L1 expression <1%, HIV-positive and locally recurrent disease, Martinelli believes that due to the small patient numbers, these subgroup data should not form the basis of deciding treatment eligibility for the retifanlimab combination.
The survival findings observed with retifanlimab plus chemotherapy are boosted by the previously reported high overall response rate of 55.8% (Lancet. 2025;405:2144–2152). As Martinelli explains, “SCAC is a very symptomatic disease, which often involves considerable bleeding and pain. Tumour shrinkage can provide marked benefit to patients’ quality of life.” She concludes, “Using chemotherapy to prime the immune system and enhance sensitivity to immunotherapy is a logical strategy in SCAC, particularly given the success observed in other HPV-related tumour types, and the POD1UM-303/InterAACT-2 trial shows conclusively that this approach works.”
Programme details:
Rao S, et al. Survival outcomes in POD1UM-303/InterAACT-2: A phase III study of retifanlimab (R) + carboplatin-paclitaxel (CP) in first-line (1L) advanced squamous anal cancer (SCAC). ESMO Immuno-Oncology Congress 2025 - Abstract 123MO