Results from two trials could expand current options for patients with EGFR or HER2-activating mutations progressing on tyrosine kinase inhibitors
As reported at the European Lung Cancer Congress 2025 (Paris, 26–29 March), phase II trials show encouraging activity with novel tyrosine kinase inhibitors (TKIs) in previously treated non-small cell lung cancer (NSCLC) with EGFR mutations or HER2-activating mutations.
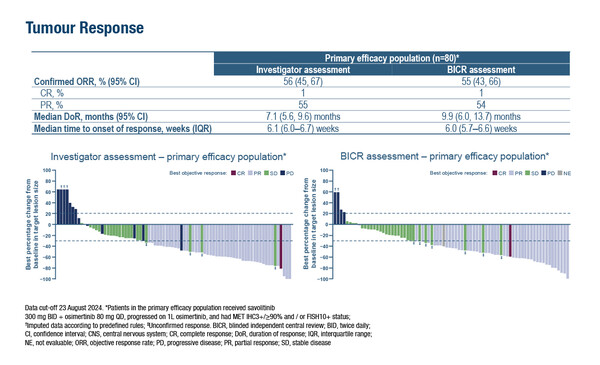
Primary results from the phase II SAVANNAH study demonstrated an investigator-assessed objective response rate (ORR) of 56% with savolitinib plus osimertinib among 80 patients with EGFR-mutated NSCLC and with MET overexpression and/or amplification following progressive disease on first-line osimertinib (Abstract 2O). Patients had MET immunohistochemistry (IHC) 3+ in ≥90% of tumour cells or fluorescence in situ hybridisation (FISH) 10+ (≥10 MET gene copies). The median duration of response (DOR) was 7.1 months and median progression-free survival (PFS) was 7.4 months.
Grade ≥3 adverse events (AEs) were reported by over half (57%) of patients in the safety analysis set, most commonly peripheral oedema (11%), increased alanine aminotransferase (6%) and pneumonia (5%). AEs resulted in discontinuation of savolitinib or osimertinib in 16% and 12% of patients, respectively.
“With the expanding number of treatment options post-osimertinib, an important consideration is how to rationalise treatment choices in the second-line setting and whether a biomarker-selected or biomarker-agnostic approach is preferred,” says Assistant Prof. Stephanie P.L. Saw from the National Cancer Centre Singapore, Singapore. “The promising results of the SAVANNAH trial certainly lend support to the approach of combining osimertinib with another matched targeted therapy based on the acquired resistance mechanism.” The combination of savolitinib plus osimertinib is now being compared with platinum-based doublet therapy in the ongoing phase III SAFFRON study (NCT05261399), and multiple treatments are being investigated in the phase II ORCHARD trial (NCT03944772), which will help to further inform optimal post-osimertinib treatment strategies for EGFR-mutated NSCLC.
In Paris, positive results were also reported from two expansion cohorts of the phase I/II SOHO-01 study investigating BAY 2927088 in previously treated patients with NSCLC harbouring HER2-activating mutations (Abstract 3O). Among 44 patients who had progressed after ≥1 systemic therapy but were naïve to HER2-targeted therapy, the ORR was 70.5% and median DOR was 8.7 months over a median follow-up of 17.2 months. In 34 patients who had previously received HER2-targeted antibody–drug conjugates (ADCs), ORR was 35.3% and median DOR was 9.5 months over a median follow-up of 10.3 months. The most common grade ≥3 treatment-related AE was diarrhoea, which occurred in 25% of patients naïve to HER2-targeted therapy and 6% of patients who had received a prior HER2-targeted ADC. Overall, treatment-related AEs led to dose reductions in 24 patients (30.8%) and treatment discontinuation in 4 patients (5.1%).
“The duration of the responses with BAY 2927088 in these heavily pre-treated patients who have limited treatment options is impressive, in both cohorts, with a manageable toxicity profile,” observes Saw. “Trastuzumab deruxtecan, a HER2-directed ADC, is the only approved therapy in the post-progression setting for patients with HER2-mutated NSCLC, but data from SOHO-01 and also the Beamion LUNG-1 trial with zongertinib (ESMO Open. 2024;9:102613) show the promising efficacy of novel HER2 TKIs in this patient population. We await the results of ongoing trials in the first-line and post-progression settings to inform the optimum sequencing approach.”
Programme details
Ahn M-J, et al. SAVANNAH: Savolitinib (savo) + osimertinib (osi) in patients (pts) with EGFRm advanced NSCLC and MET overexpression (OverExp) and/or amplification (Amp) following progressive disease (PD) on osi. European Lung Cancer Congress 2025, Abstract 2O
Proffered Paper Session 1, 26.03.2025, h. 16:45 – 18:15, Room South Paris
Girard N, et al. Phase I/II SOHO-01 study of BAY 2927088 in patients with previously treated HER2-mutant NSCLC: Safety and efficacy results from 2 expansion cohorts. European Lung Cancer Congress 2025, Abstract 3O
Proffered Paper Session 1, 26.03.2025, h. 16:45 – 18:15, Room South Paris